Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. Does a precipitate form when A and B empirical formula of precipitate solution A solution B are mixed? lead(II) nitrate potassium iodide O yes O no dlo copper(II) bromide sodium sulfide O yes Periodic Table no manganese(II) bromide zinc acetate O yes O no
Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. Does a precipitate form when A and B empirical formula of precipitate solution A solution B are mixed? lead(II) nitrate potassium iodide O yes O no dlo copper(II) bromide sodium sulfide O yes Periodic Table no manganese(II) bromide zinc acetate O yes O no
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 39P
Related questions
Question
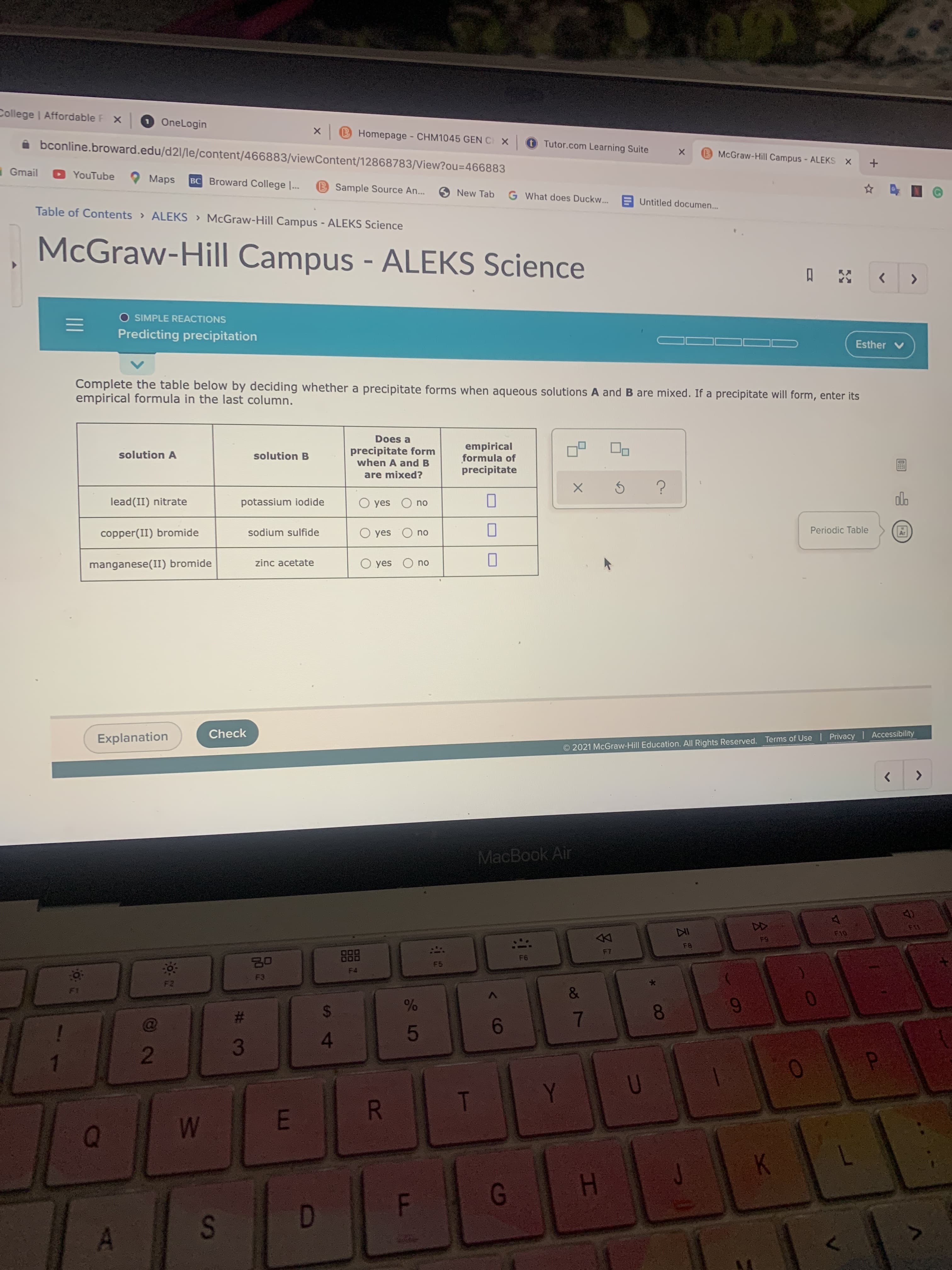
Transcribed Image Text:Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its
empirical formula in the last column.
Does a
precipitate form
when A and B
empirical
formula of
precipitate
solution A
solution B
are mixed?
lead(II) nitrate
potassium iodide
O yes O no
dlo
copper(II) bromide
sodium sulfide
O yes
Periodic Table
no
manganese(II) bromide
zinc acetate
O yes O no
Expert Solution
Step 1 up
when the aqueous solution of potassium iodide and aqueous solution lead nitrate, mixed together, a yellow precipitate of lead iodide [PbI2(s)] and water-soluble KNO3 will form.
It’s an example of a double displacement reaction.
2KI(aq)+Pb(NO3)2(aq)→PbI2(s)+2KNO3
Does a precipitate form [yes]
Empirical formula PbI2
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning