Complete the table below for calculating the formula weight of the ionic compound zinc sulfide, ZnS. (Enter atomic and formula weights to two decimal places.) Atomic Number Weight of Ions Ion Weight of Ions Zn2+ amu amu S2- amu amu Formula weight zinc sulfide amu
Complete the table below for calculating the formula weight of the ionic compound zinc sulfide, ZnS. (Enter atomic and formula weights to two decimal places.) Atomic Number Weight of Ions Ion Weight of Ions Zn2+ amu amu S2- amu amu Formula weight zinc sulfide amu
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 85E: There are two binary compounds of mercury and oxygen. Heating either of them results in the...
Related questions
Question
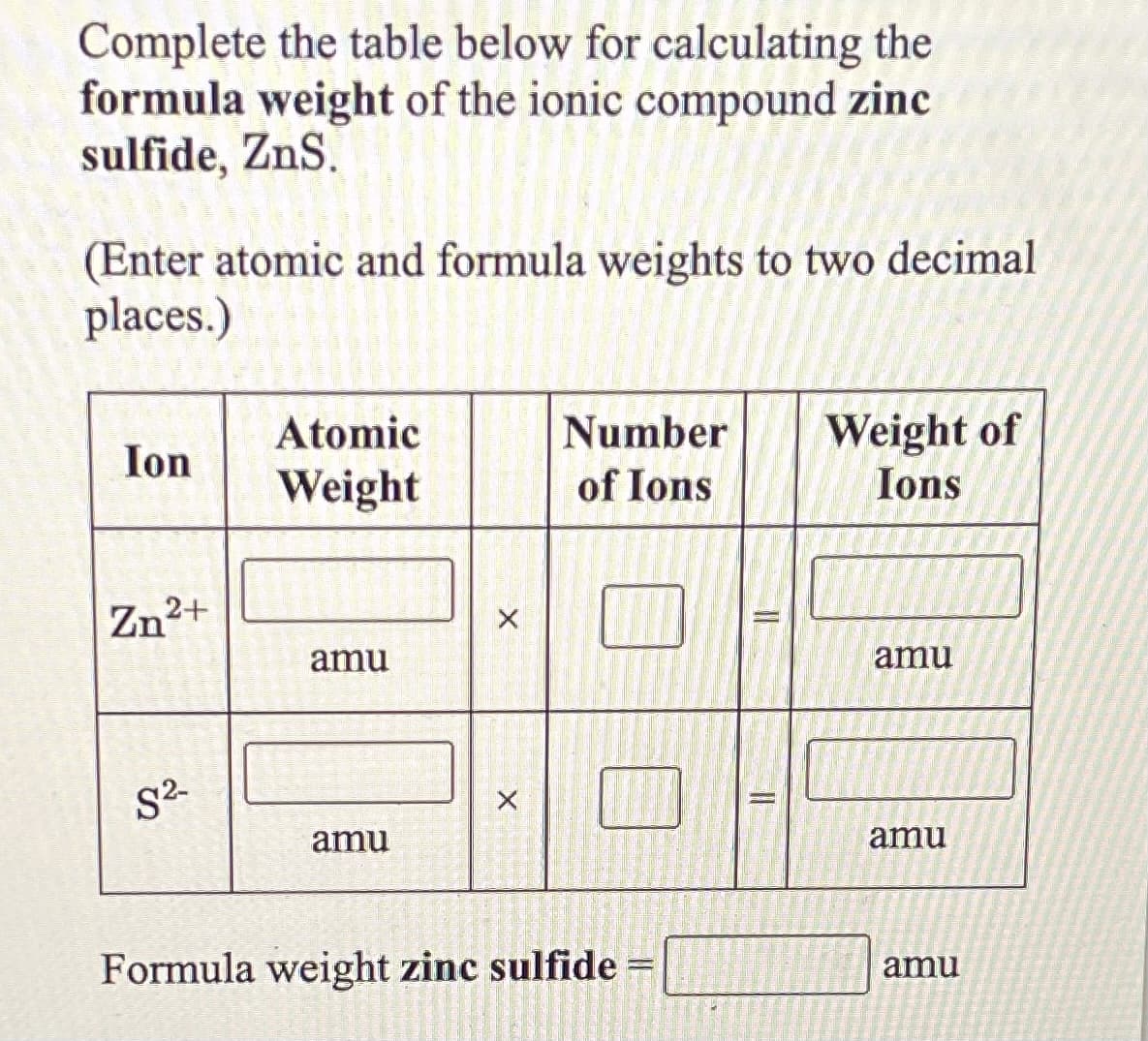
Transcribed Image Text:Complete the table below for calculating the
formula weight of the ionic compound zinc
sulfide, ZnS.
(Enter atomic and formula weights to two decimal
places.)
Ion
Atomic
Number
Weight of
Weight
of Ions
Ions
Zn2+
amu
amu
S2
amu
amu
Formula weight zinc sulfide =
amu
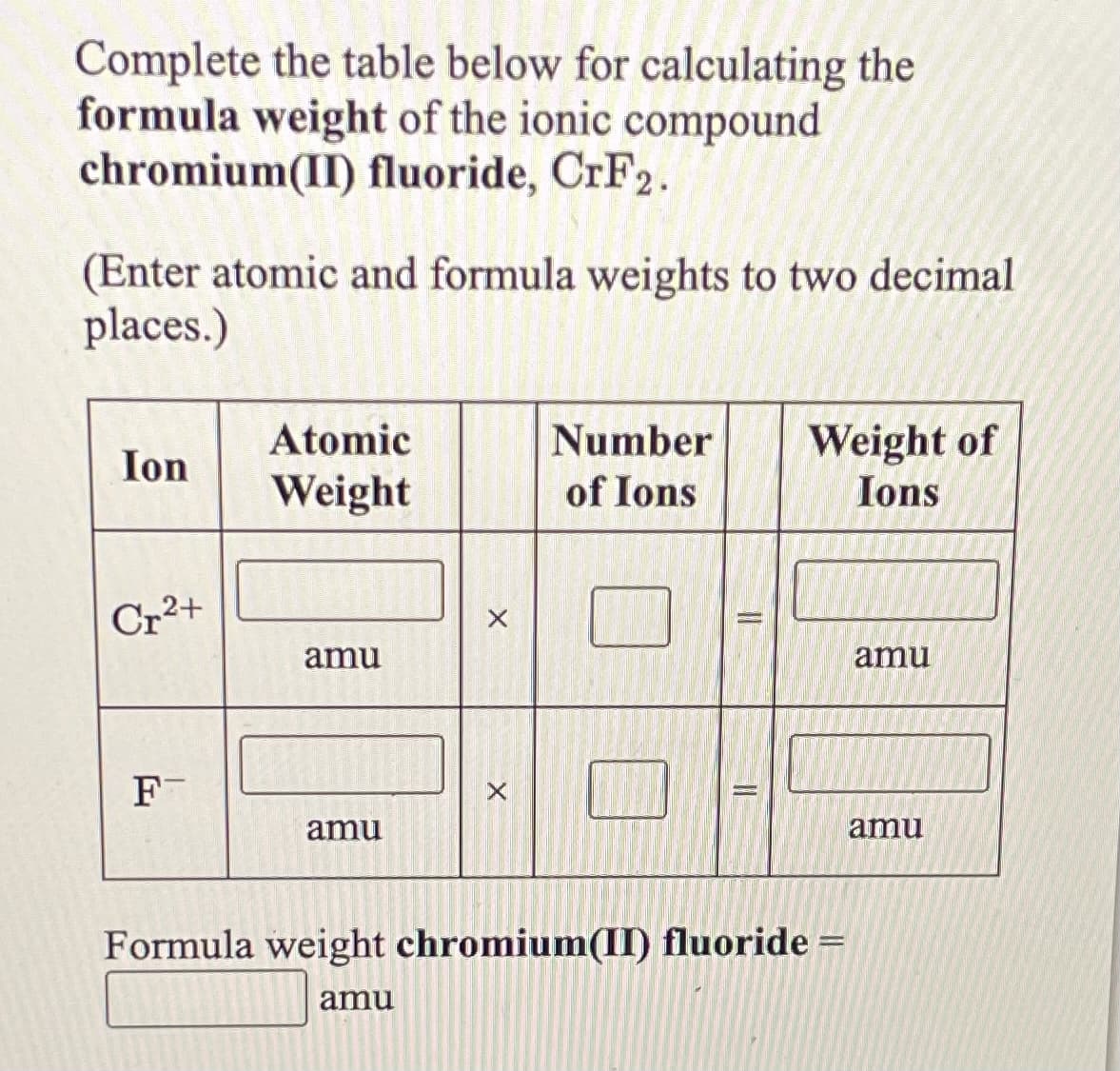
Transcribed Image Text:Complete the table below for calculating the
formula weight of the ionic compound
chromium(II) fluoride, CrF2.
(Enter atomic and formula weights to two decimal
places.)
Atomic
Number
Weight of
Ions
Ion
Weight
of Ions
Cr2+
amu
amu
F
amu
amu
Formula weight chromium(II) fluoride =
amu
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning