Complete the table below, using the diagram of an atom shown at right. A Properties of subatomic particles approximate charge (in multiples of e) location name symbol mass on diagram (amu) 1.0 A +1 1.0 A neutron |(choose one)| (choose one)
Complete the table below, using the diagram of an atom shown at right. A Properties of subatomic particles approximate charge (in multiples of e) location name symbol mass on diagram (amu) 1.0 A +1 1.0 A neutron |(choose one)| (choose one)
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter2: Atoms
Section: Chapter Questions
Problem 2.89P: 2-89 Assume that a new element has been discovered with atomic number 117. Its chemical properties...
Related questions
Question
100%
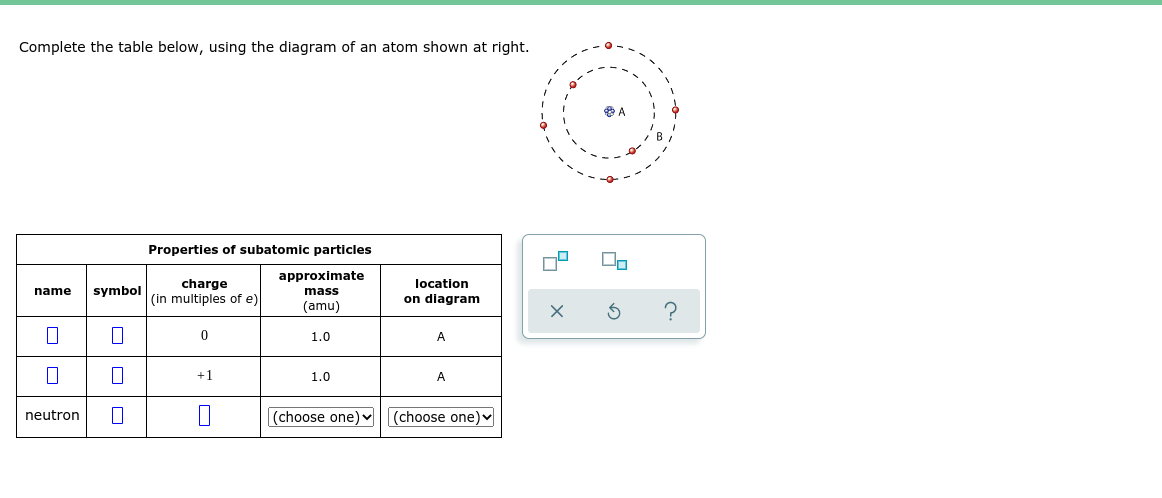
Transcribed Image Text:Complete the table below, using the diagram of an atom shown at right.
Properties of subatomlc particles
approximate
charge
(in multiples of e)
location
name
symbol
mass
on diagram
(amu)
1.0
A
+1
1.0
A
neutron
|(choose one)
(choose one)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co