Diffusion of alcohol through water and N2.
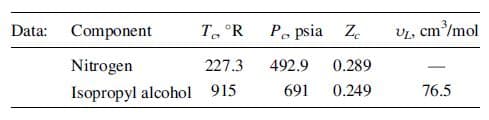
Isopropyl alcohol undergoes mass transfer at 35oC and 2 atm under dilute conditions through water, across a phase boundary, and then through nitrogen. Based on the data given below, estimate for isopropyl alcohol: (a) the diffusivity in water using the Wilke–Chang equation; (b) the diffusivity in nitrogen using the Fuller et al. equation; (c) the product, DABρM, in water; and (d) the product, DABρM, in air, where ρM is the mixture molar density.Compare: (e) the diffusivities in parts (a) and (b); (f) the results from parts (c) and (d). (g) What do you conclude about molecular diffusion in the liquid phase versus the gaseous phase?