The figure below shows part of a gas distribution system. In this process, all valves are linear and have an equal resistance 'R', the two tanks have constant equal volumes 'V', and the process is isothermal. Under the process conditions the gas behaves as an ideal gas. The mass flow rate through any of the valves is proportional to the pressure difference across the valve. Both tanks discharge against atmospheric pressure Pa' desired to study the dynamics of gas distribution in this process as Pii and Piz are changed. Initially Pi = Pi2 =2 atm. 1 atm. It is %3D %3D Piz Pa ever P4 P, ,Pa Pa Pa Piz P2 Perform the following tasks: a) Identify the state variables and inputs of this process. b) Develop a transient model for this process. c) Calculate the value of the state variables at the initial steady. d) Express the density of the gas in terms of the pressure, temperature, molecular weight of the gas M, and Rg the ideal gas constant. e) Obtain a Laplace domain representation of the model. f) Obtain transfer functions relating P1(s) to both Pin(s) and Pi2(s). And the same for P2(s). g) What is the value of the second order damping coefficient (C) for this process? h) If Pn is suddenly increased to 3 atm (while Piz is kept constant), what are the new values of Pi and P2 that will result after enough time has passed? i) Show that, at the new steady state, 60% of the increase in mass flow in the feed to tank 1 leaves in the exit from tank 1, and 20% leaves in the exit from tank 2. What happens to the remaining 20%?
The figure below shows part of a gas distribution system. In this process, all valves are linear and have an equal resistance 'R', the two tanks have constant equal volumes 'V', and the process is isothermal. Under the process conditions the gas behaves as an ideal gas. The mass flow rate through any of the valves is proportional to the pressure difference across the valve. Both tanks discharge against atmospheric pressure Pa' desired to study the dynamics of gas distribution in this process as Pii and Piz are changed. Initially Pi = Pi2 =2 atm. 1 atm. It is %3D %3D Piz Pa ever P4 P, ,Pa Pa Pa Piz P2 Perform the following tasks: a) Identify the state variables and inputs of this process. b) Develop a transient model for this process. c) Calculate the value of the state variables at the initial steady. d) Express the density of the gas in terms of the pressure, temperature, molecular weight of the gas M, and Rg the ideal gas constant. e) Obtain a Laplace domain representation of the model. f) Obtain transfer functions relating P1(s) to both Pin(s) and Pi2(s). And the same for P2(s). g) What is the value of the second order damping coefficient (C) for this process? h) If Pn is suddenly increased to 3 atm (while Piz is kept constant), what are the new values of Pi and P2 that will result after enough time has passed? i) Show that, at the new steady state, 60% of the increase in mass flow in the feed to tank 1 leaves in the exit from tank 1, and 20% leaves in the exit from tank 2. What happens to the remaining 20%?
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Please do a,b,c
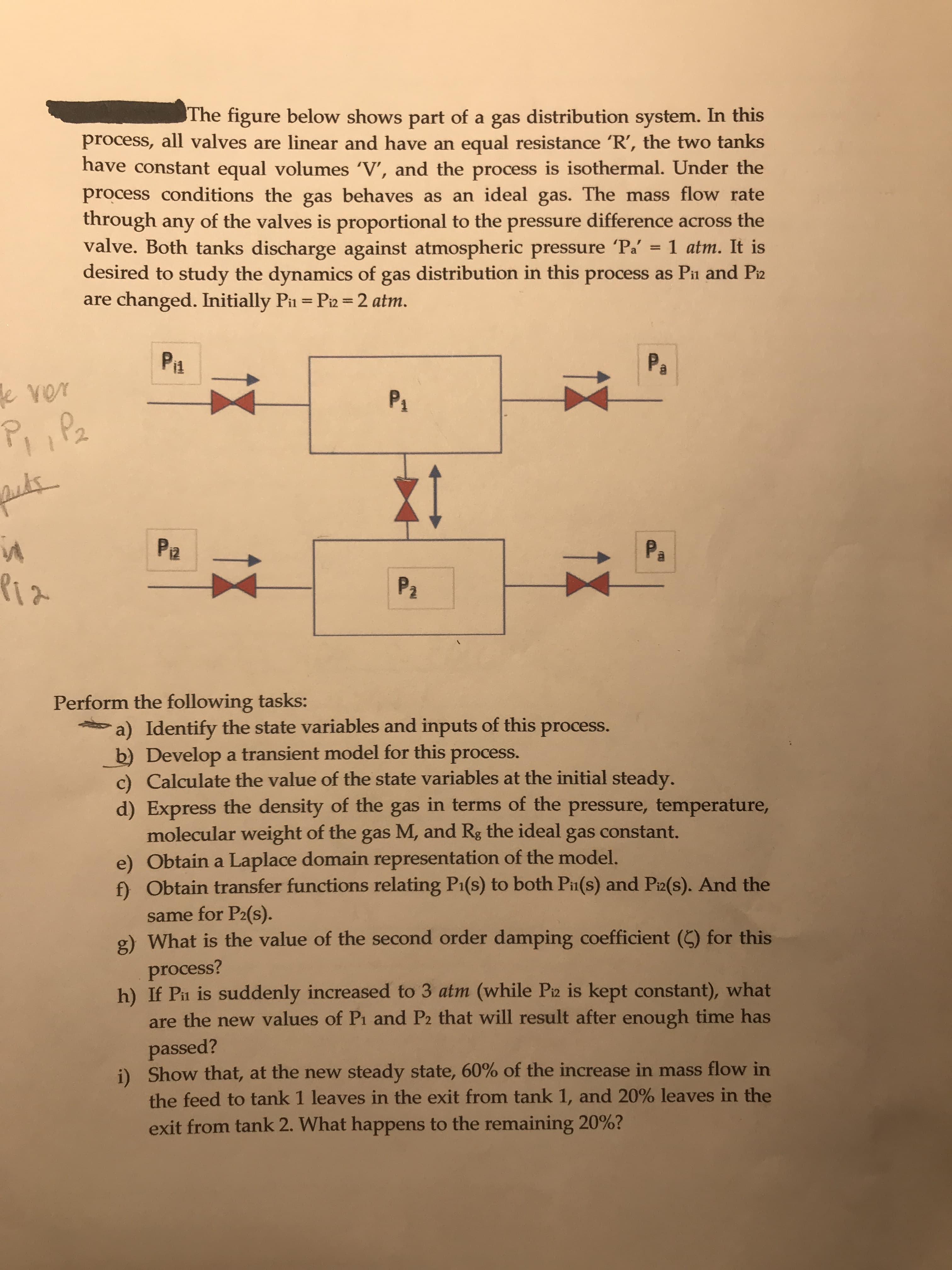
Transcribed Image Text:The figure below shows part of a gas distribution system. In this
process, all valves are linear and have an equal resistance 'R', the two tanks
have constant equal volumes 'V', and the process is isothermal. Under the
process conditions the gas behaves as an ideal gas. The mass flow rate
through any of the valves is proportional to the pressure difference across the
valve. Both tanks discharge against atmospheric pressure Pa'
desired to study the dynamics of gas distribution in this process as Pii and Piz
are changed. Initially Pi = Pi2 =2 atm.
1 atm. It is
%3D
%3D
Piz
Pa
ever
P4
P, ,Pa
Pa
Pa
Piz
P2
Perform the following tasks:
a) Identify the state variables and inputs of this process.
b) Develop a transient model for this process.
c) Calculate the value of the state variables at the initial steady.
d) Express the density of the gas in terms of the pressure, temperature,
molecular weight of the gas M, and Rg the ideal gas constant.
e) Obtain a Laplace domain representation of the model.
f) Obtain transfer functions relating P1(s) to both Pin(s) and Pi2(s). And the
same for P2(s).
g) What is the value of the second order damping coefficient (C) for this
process?
h) If Pn is suddenly increased to 3 atm (while Piz is kept constant), what
are the new values of Pi and P2 that will result after enough time has
passed?
i) Show that, at the new steady state, 60% of the increase in mass flow in
the feed to tank 1 leaves in the exit from tank 1, and 20% leaves in the
exit from tank 2. What happens to the remaining 20%?
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 10 steps with 12 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The