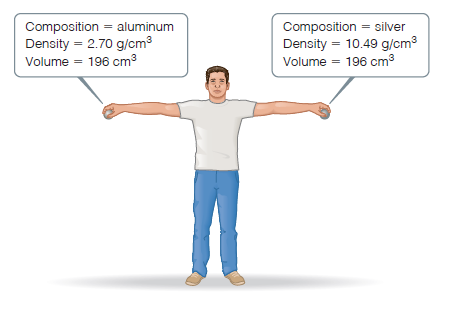
Consider the two spheres shown here, one made of silver and
the other of aluminum. (a) What is the mass of each sphere
in kg? (b) The force of gravity acting on an object is F = mg,
where m is the mass of an object and g is the acceleration of
gravity (9.8 m/s2). How much work do you do on each sphere
it you raise it from the floor to a height of 2.2 m? (c) Does the
act of lifting the sphere off the ground increase the potential
energy of the aluminum sphere by a larger, smaller, or
same amount as the silver sphere? (d) If you release the
spheres simultaneously, they will have the same velocity
when they hit the ground. Will they have the same kinetic
energy? If not, which sphere will have more kinetic energy?
[Section 1.4]
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 5 images








