composition of water in a hydrate, we need to look at our equation. compare it to your experimental values you just obtained in lab. To calculate the percent In this exercise, we are going to calculate the percent composition of water in a hydrate and percent (%) composition = of water mass of water х 100 mass of entire hydrate For LINO3 3H20 (lithium nitrate trihydrate) we have to calculate the mass of each element. Nelice the three in front of the water will give us a total of six hydrogens and three oxygens. Li: 1 x 6.94 g/mol N: 1x 14.01 g/mol H: 6x 1.01 g/mol O: 3x 16.00 g/mol O: 3x 16.00 g/mol 19ot lo0 68.95 g/mol a bluow e 54.06 g/mol percent (%) composition = of water 3 H20 х 100 LINO, · 3H20 percent (%) composition = of water 54.06 g/mol х 100 (68.95 g/mol + 54.06 g/mol) percent (%) composition of water in LINO3 • 3 H20 = 43.9 % 1. Calculate the percent composition of water in the following hydrates: (show all work) ZnSO, 7H20 MgSO, 7H20 BaCl2 · 2H20 There are additional questions on the other side.
composition of water in a hydrate, we need to look at our equation. compare it to your experimental values you just obtained in lab. To calculate the percent In this exercise, we are going to calculate the percent composition of water in a hydrate and percent (%) composition = of water mass of water х 100 mass of entire hydrate For LINO3 3H20 (lithium nitrate trihydrate) we have to calculate the mass of each element. Nelice the three in front of the water will give us a total of six hydrogens and three oxygens. Li: 1 x 6.94 g/mol N: 1x 14.01 g/mol H: 6x 1.01 g/mol O: 3x 16.00 g/mol O: 3x 16.00 g/mol 19ot lo0 68.95 g/mol a bluow e 54.06 g/mol percent (%) composition = of water 3 H20 х 100 LINO, · 3H20 percent (%) composition = of water 54.06 g/mol х 100 (68.95 g/mol + 54.06 g/mol) percent (%) composition of water in LINO3 • 3 H20 = 43.9 % 1. Calculate the percent composition of water in the following hydrates: (show all work) ZnSO, 7H20 MgSO, 7H20 BaCl2 · 2H20 There are additional questions on the other side.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 86AP: Consider a hypothetical compound composed of elements X, Y, and Z with the empirical formula X2YZ3 ....
Related questions
Question
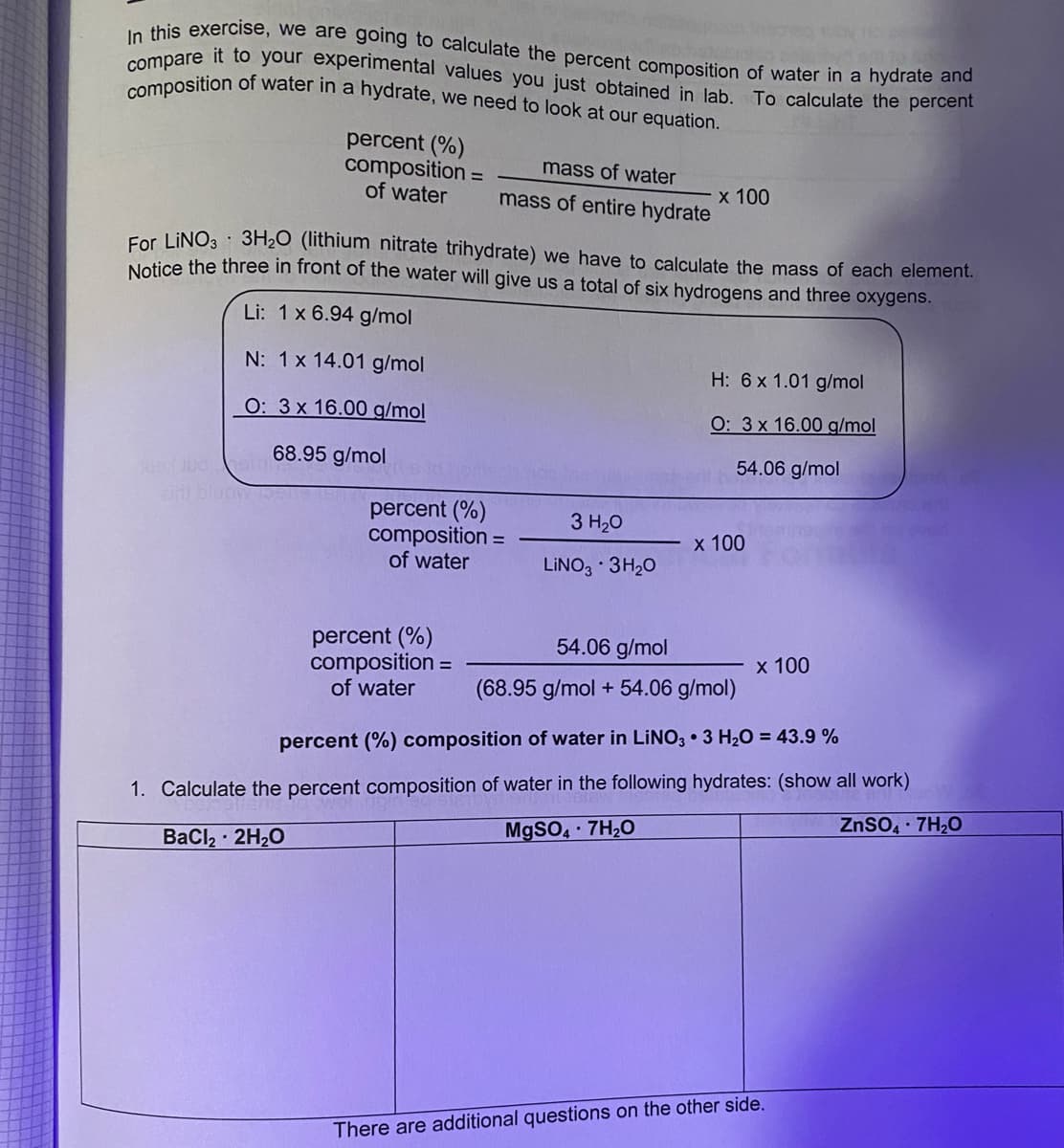
Transcribed Image Text:composition of water in a hydrate, we need to look at our equation.
compare it to your experimental values you just obtained in lab. To calculate the percent
In this exercise, we are going to calculate the percent composition of water in a hydrate and
percent (%)
composition =
of water
mass of water
х 100
mass of entire hydrate
For LINO3 3H2O (lithium nitrate trihydrate) we have to calculate the mass of each element.
Nelice the three in front of the water will give us a total of six hydrogens and three oxygens.
Li: 1x 6.94 g/mol
N: 1x 14.01 g/mol
H: 6x 1.01 g/mol
О: 3х 16.00 g/mol
O: 3x 16.00 g/mol
54.06 g/mol
go l00 68.95 g/mol
percent (%)
composition =
of water
3 H20
х 100
LINO3 · 3H20
percent (%)
composition =
of water
54.06 g/mol
х 100
(68.95 g/mol + 54.06 g/mol)
percent (%) composition of water in LINO3 • 3 H20 = 43.9 %
1. Calculate the percent composition of water in the following hydrates: (show all work)
ZnSO, 7H20
M9SO 7H20
BaCl2 2H20
There are additional questions on the other side.
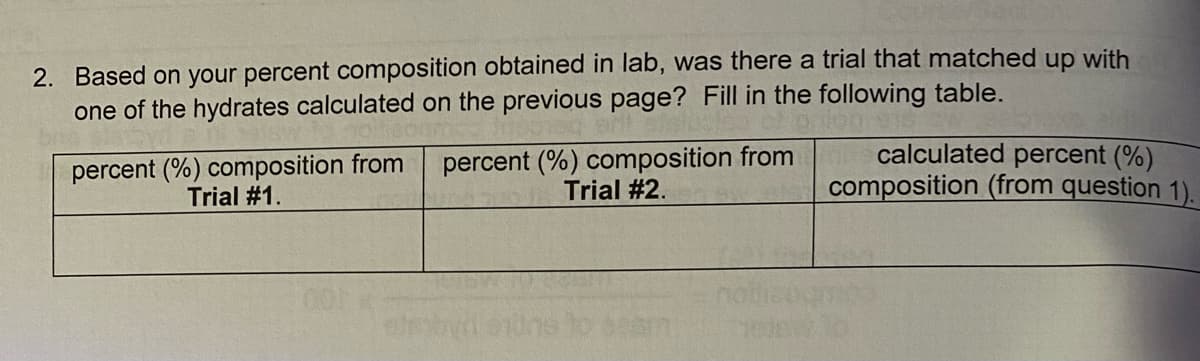
Transcribed Image Text:2. Based on your percent composition obtained in lab, was there a trial that matched up with
one of the hydrates calculated on the previous page? Fill in the following table.
percent (%) composition from
Trial #1.
percent (%) composition from
Trial #2.
calculated percent (%)
composition (from question 1).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning