name & geometries. F3CI Molecule or lon (circle) # of bonding regions= # of non-bonding pairs= VSEPR formula hybridization Electronic geometry# of bonding regions= Molecular geometry Polar or nonpolar (circle) 2S Molecule or lon (circle) # of bonding regions= # of non-bonding pairs= VSEPR formula hybridization Electronic geometry# of bonding regions=
name & geometries. F3CI Molecule or lon (circle) # of bonding regions= # of non-bonding pairs= VSEPR formula hybridization Electronic geometry# of bonding regions= Molecular geometry Polar or nonpolar (circle) 2S Molecule or lon (circle) # of bonding regions= # of non-bonding pairs= VSEPR formula hybridization Electronic geometry# of bonding regions=
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 70P: Ozone (O3) has a nonzero dipole moment. In the molecule of O3 , one of the oxygen atoms is directly...
Related questions
Question
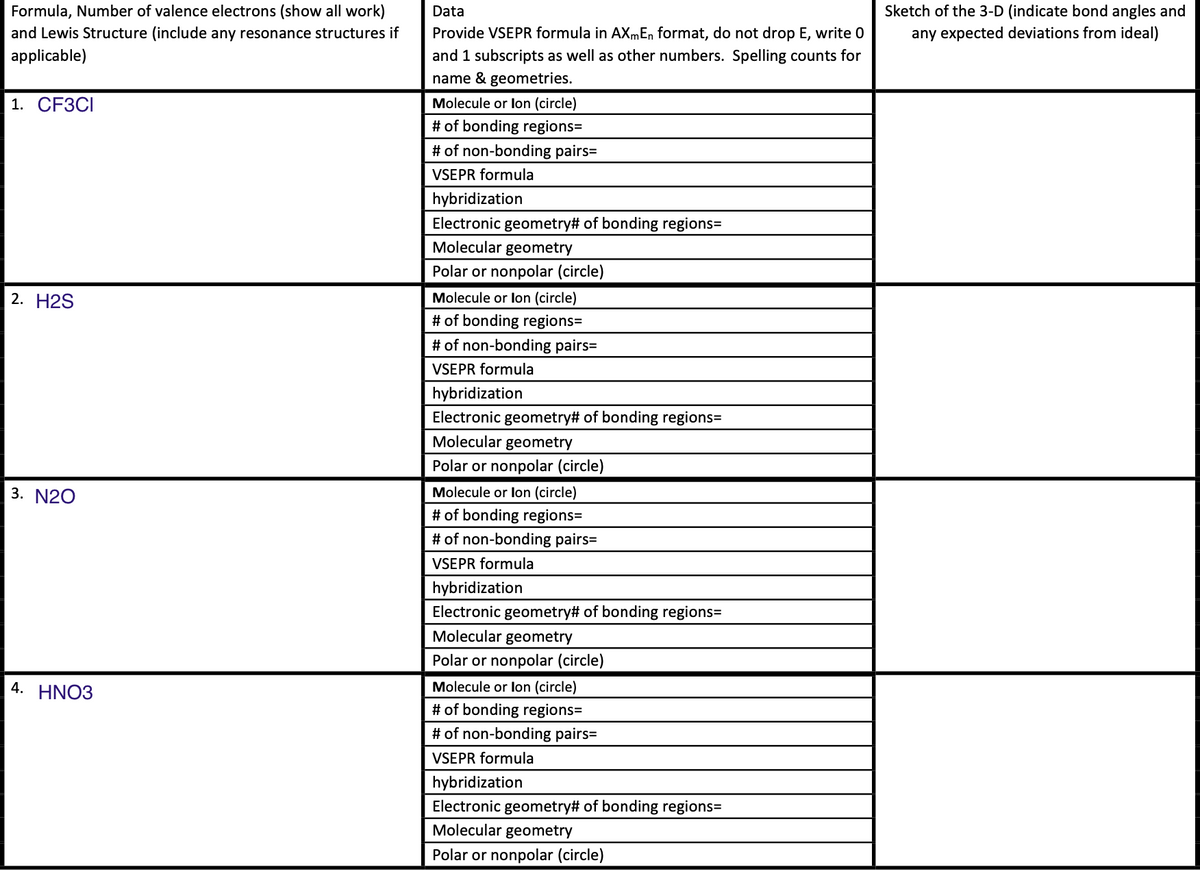
Transcribed Image Text:Formula, Number of valence electrons (show all work)
Sketch of the 3-D (indicate bond angles and
any expected deviations from ideal)
Data
and Lewis Structure (include any resonance structures if
Provide VSEPR formula in AXmEn format, do not drop E, write 0
applicable)
and 1 subscripts as well as other numbers. Spelling counts for
name & geometries.
1. СЕЗСІ
Molecule or lon (circle)
# of bonding regions=
# of non-bonding pairs=
VSEPR formula
hybridization
Electronic geometry# of bonding regions=
Molecular geometry
Polar or nonpolar (circle)
2. Н2S
Molecule or lon (circle)
# of bonding regions=
# of non-bonding pairs=
VSEPR formula
hybridization
Electronic geometry# of bonding regions=
Molecular geometry
Polar or nonpolar (circle)
3. N20
Molecule or lon (circle)
# of bonding regions=
# of non-bonding pairs=
VSEPR formula
hybridization
Electronic geometry# of bonding regions=
Molecular geometry
Polar or nonpolar (circle)
4. HNO3
Molecule or lon (circle)
# of bonding regions=
# of non-bonding pairs=
VSEPR formula
hybridization
Electronic geometry# of bonding regions=
Molecular geometry
Polar or nonpolar (circle)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning