Q: Which of the following is the strongest acid? Fluorine is bolded for contrast. 0 H3C 0000 О А ОВ D…
A: Which of the following is strongest acid ?
Q: (a) Rank the following compounds in order of increasing acidity. (b) Which compound forms the…
A: The acidic strength of the organic acids is determined by the stability of the corresponding…
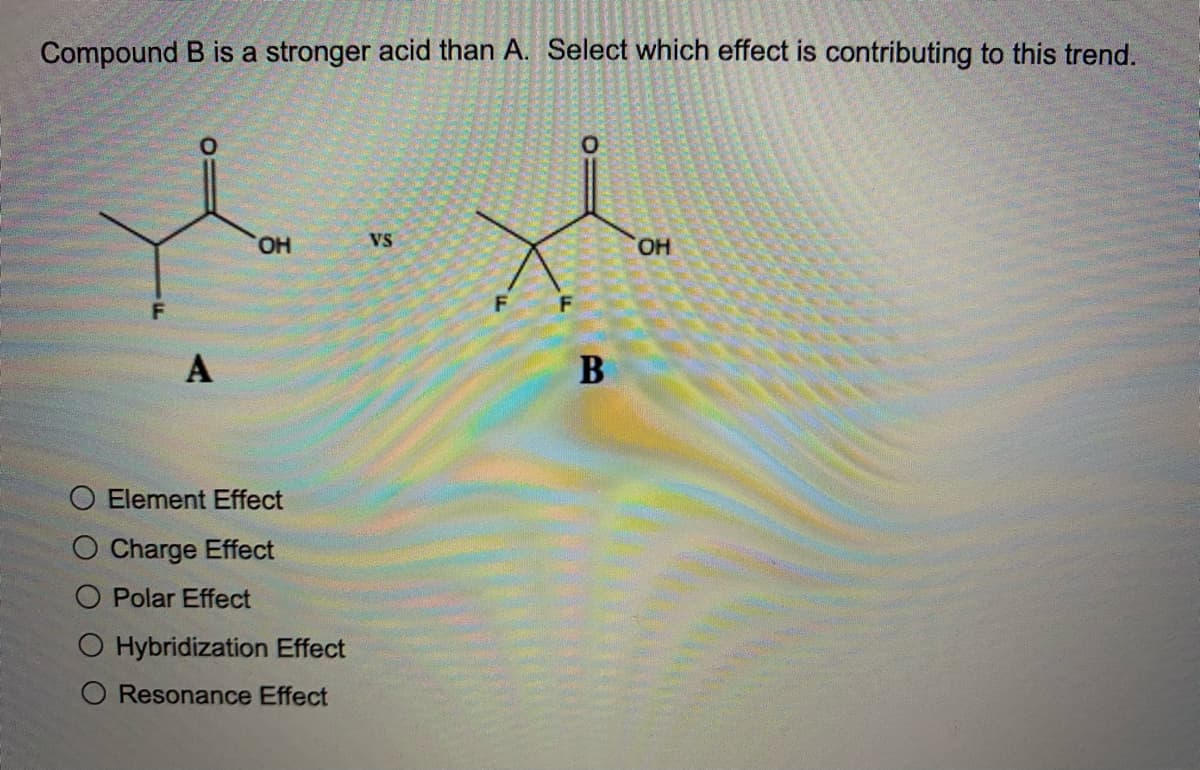
Q: Without reference to a pKa table, decide which compound in each pair is the stronger acid.
A: a. When we talk about the acidity of the of the elements we consider the periodic table which we can…
Q: Which compound in the following pair is the stronger acid? Select the single best answer. Br HO.
A: According to Arrhenius theory of acid and base. An acid is a compound which releases a proton and…
Q: 1. For each pair, predict which is the stronger base. Explain ОН HO or or IZ
A:
Q: What is the weaker acid in the following reaction if the equilibrium lies to the right? NH2 HC=C NH3…
A: In equilibrium condition acid donate protons and form its conjugate base whereas base accepts proton…
Q: Which is a stronger base, PO4 3- or AsO4 3-? Explain.
A: An acid is a substance that gives H+ ions in its solution whereas a basic substance gives OH- ions…
Q: In attached file, Which compound in each pair is the stronger acid?
A: The acidity of carboxylic acid is increases, when it contains electron withdrawing group is near in…
Q: Which of the attached anions is the stronger base? Explain your choice.
A: INTRODUCTION: Lewis base: The species that contain (-ve) charge act as lewis base.Molecules that…
Q: In each row of the table, select the stronger acid or stronger base, as instructed. HO equally…
A: Acids are those substance which donates H+ ion. The strength of acid can be compared on the basis of…
Q: In HNO3 + H2O —> H3O+ + NO3- , who is the Bronsted base?
A:
Q: For each reaction, label the Lewis acid and base. Use curved arrow notation to show the movement of…
A: Lewis acid is that compound which accepts a lone pair of electrons And Lewis base is that compound…
Q: In each row of the table, select the stronger acid or stronger base, as instructed. N. N. equally…
A: Acid is a substance which can accept a lone pair of electrons while base is the one which can donate…
Q: Which compound of each of the following pairs is the STRONGER ACID? * (A) (B) O Option A O Option B…
A:
Q: ollowing compounds, rank in order of acidity ( the most acidic being 1) ain the reason for your…
A: The first one in the very first image is: C6H5CH2CH3 and let it be (A) Second one in the first…
Q: Without reference to a pKa table, decide which compound in each pair is the stronger acid. (see…
A:
Q: (a) Which of the following acids is most acidic? (strongest acid has the most stable conjugate…
A:
Q: Which would be the stronger Bronsted acid, the molecule on the left or the molecule on the right?…
A:
Q: | Rank the following compounds from strongest acid to weakest acid. `OH F3C *OH A D
A: Carboxylic acids and ketones both belong to the class of carbonyl group. But carboxylic acids are…
Q: Which acid is the strongest? a. HNO2 b. NH3 c. HF d. HClO4
A: Introduction : Strong acid is defined as which can donate H+ ions easily .
Q: 2. Which structure is the stronger acid, A or B? Explain your answer. F Br A B
A:
Q: 10. For each pair of molecules, circle the stronger acid. Use pka values to guide your decisions. HO…
A:
Q: If AH is a stronger acid than BH, which of the following is/are true? 1 A is a stronger base than B.…
A: Given that AH is stronger acid than BH. Based on given statement, we have to write the true…
Q: F ONLY- ( STRUCTURAL ANSWER ONLY)
A:
Q: Rank the compounds in order of decreasing acidity. Most acidic Least acidic Answer Bank BRCH, CO,H…
A:
Q: Vhich of the following is the strongest base? `NH2 (A) (B) (C) (D)
A: (D) is neither weak base nor strong base because it is a salt of ammonia called ammonium ion. (A)…
Q: Select the stronger base from each pair of compounds.
A: The stronger base has to be identified from the two.
Q: Rank the following in order of increasing basicity. The first in your list should be the least…
A: OH- is a conjugate base of H2O. F- is a conjugate base of HF. Cl- is a conjugate base of HCl.
Q: Rank the following conjugate bases in order of decreasing basicity, putting the most basic first.…
A: The basic strength of any molecule is directly proportional to the tendency to donate the lone pair…
Q: 1. (2) Circle the strongest acid. a) HC;H5O2 Ka= 6.3 x 105 b) HCN Ka = 4.9 x 10-10 c) HNO2 pKa =…
A: We know that :- Ka is acid dissociation constant. More Ka value, strong is the acid. And pKa =…
Q: In a triprotic acid, which Ka has the highest value? Select one: O a. Ka1 O b. Ka2 О с. Каз O d. Kb1…
A: A protic acid is the one which contains protons .There are diprotic acids, triprotic etc, or…
Q: 1. Which is the stronger acid between: Compound A - pKa is 10 Compound B - ka is 1.5 × 10^-16
A: Given information, Compound A - pKa is 10 Compound B - ka is 1.5 × 10^-16
Q: Is NH2 stronger or weaker base than OH-? explain why?
A: Answer: NH2 - is stronger base than OH- Explanation: Proton donar is Bronsted acid . Weak acid…
Q: i need help to rank each 5 compunds by relative basicity use 1 for the strongest base all the way 5…
A:
Q: 1) Which is the stronger acid: a) NH4 or H2O b) BrCH2- OH or FCH2-OH c) H2SO4 or H2SO3
A:
Q: For the following sets of substances, pick che correct one and add an explanation. a) the weakest…
A: Soln
Q: Which factors affect the strength of acid? a) The polarity of the molecule b) The strength of the…
A: Stronger acid is the acid in which proton can be easily donated.
Q: Which of the following is the strongest base? F− ClO4− NO3− Cl− H2O
A: When an acid donates a proton, it becomes a base, and when a base accepts a proton, an acid is…
Q: Using the Identity of X in HX to Determine Relative Acidity Without reference to a pKa table, decide…
A: Since F is more electronegative than O, the H-F bond is more polar than H-O bond. The H-F bond…
Q: interest are: phenol (pK, = 9.9), and acetic acid (pK, = 4.8). LOH CH3- CH3- он A в D acetate phenol…
A:
Q: 14 HC it ○ニ エ 土
A: Acidity is defined as the ease with which a molecule can donate protons. if it can donate PROTONS…
Q: Which anion (A or B) is the stronger base?
A: A base is a substance which is capable of accepting H+ ions or donating electron pairs. The ease…
Q: Part E. Choose the More Acidic for Each of the Following Pairs and Explain why! NH3 ONH4 1. OH2 `NH2…
A:
Q: Which compound of each of the following pairs is the STRONGER ACID? (A) (B) O Option A O Option B…
A: Given, Which compound of each of the following pairs is the stronger acid, A) H2SO4 or B) H2SO3…
Q: c. List the given compounds in order of decreasing basicity. Give a reason for your answer. i. : NH3…
A: Nitrogen is less electronegative than oxygen and has ability to donate its electron pair
Q: order from strongest acid to weakest acid. H* HC H* H* B' HC НА D HB A D НА H* HC B' HD НА HC A H* D…
A: We are given four containers with weak acids HA, HB, HC and HD. we need to arrange them in order of…
Q: Predict the stronger acid in each pair: (a) HNO3 or HNO2;(b) H2S or H2O; (c) H2SO4 or H2SeO4; (d)…
A: INTRODUCTION: Acid is defined as a substance which releases H+ ions according to bronsted acid-base…
Q: Part I. Choose the stronger acid in each pair of compounds. 1. H,Se and H2S 2. H3P and H2S 3. HBrO2…
A: Disclaimer: Since you have posted a question with multiple sub-parts, we will solve the first three…
Q: (b) Which of the following bases is most basic? (strongest base has the most stable conjugate acid).…
A:
Q: Rank the compounds in each group in order of increasing acidity.
A: Acidity gets measured by observing any compound's H+ releasing ability. The more facilely any…
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- What are the Step to be followed to determining acidity using hybridization effects ?Calculate the strength (pKa) of the selenium oxoacids as stated below, thus determine which one is the strongest acid. i. H2SeO4ii. H2SeO31) How do sulfur trioxide and sulfite similar? How do they differ? 2) If you dissolved 1 mole of each (sulfur trioxide and sulfite) in a liter of water, which would give you a solution with a lower pH?
- Which factors affect the strength of acid? a) The polarity of the molecule b) The strength of the H-A bond c) Stronger acids have a larger Ka d) All of the aboveThe strength of Lewis bases follows the order of... A. NH3 > Me2NH > Me3N B. Me3N > Me2NH > MeNH2 C. NH3 > MeNH2 > EtNH2 D. Me2NH > MeNH2 > NH3Answer the following questions based on nitrous acid. a) Name the hybridization of the circled N. b)Write the O–N–O bond angle. c) Name the atomic (or hybrid atomic) orbitals used to form the N=O sigma and pi bonds. d) Name the atomic (or hybrid atomic) orbitals used to form the O–H sigma bond. Answer the following questions based on formic acid. a) Name the hybridization of the circled O b) Write the O–C–O bond angle. c) Name the atomic (or hybrid atomic) orbitals used to form the C=O sigma and pi bonds. d) Name the atomic (or hybrid atomic) orbitals used to form the C–H bond sigma bond.
- Which of the statements below would correctly explain why hydrochloric acid is a stronger acid that hydrofluoric acid? a. Fluorine is higher on the periodic table than chlorine. b. Fluorine has a smaller atomic radius than chlorine. c. Fluorine has fewer electrons than chlorine. Selected:d. Fluorine has a larger electronegativity than chlorine.Take another look at answer choices B & D. These reactions show the starting species acting as a base and forming hydroxide ions when they react with water. Base reactions have corresponding Kb equations and values. Match the reactions shown in choices B & D with their corresponding Kbs (Kb1, Kb2, or Kb3).Identify the hydronium ion among the following ions. H2O2+ H2O2- H3O+ H3O- H3O2+

