Compound CH4 Boiling Point -164 °C Melting Point -182 °C -115 °C O °C Intermolecular Force London dispersion Dipole-dipole Hydrogen Bonding HCI -85 °C H20 100 °C Guided Questions: 1. Based on the results of your data, which among the liquids has a lower surface tension? 2. Based on the results of your data, how is viscosity related to intermolecular force? 3. Which compound has the highest boiling point?
Compound CH4 Boiling Point -164 °C Melting Point -182 °C -115 °C O °C Intermolecular Force London dispersion Dipole-dipole Hydrogen Bonding HCI -85 °C H20 100 °C Guided Questions: 1. Based on the results of your data, which among the liquids has a lower surface tension? 2. Based on the results of your data, how is viscosity related to intermolecular force? 3. Which compound has the highest boiling point?
Chapter22: Bulk Electrolysis: Electrogravimetry And Coulometry
Section: Chapter Questions
Problem 22.6QAP
Related questions
Question
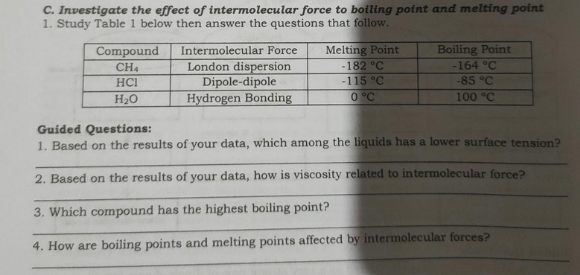
Transcribed Image Text:C. Investigate the effect of intermolecular force to bolling point and melting point
1. Study Table 1 below then answer the questions that follow.
Melting Point
-182 °C
-115 °C
0 °C
Compound
Boiling Point
Intermolecular Force
London dispersion
Dipole-dipole
Hydrogen Bonding
CH4
-164 °C
HCI
-85 °C
H20
100 °C
Guided Questions:
1. Based on the results of your data, which among the liquids has a lower surface tension?
2. Based on the results of your data, how is viscosity related to intermolecular force?
3. Which compound has the highest boiling point?
4. How are boiling points and melting points affected by intermolecular forces?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

