Consider a mixture of three different gases: 1.70 g of argon (molecular mass = 39.948 g/mol), 2.90 g of neon (molecular mass = 20.180 g/mol), and 3.70 g of helium (molecular mass = 4.0026 g/mol). For this mixture, determine the percentage of the total number of atoms that corresponds to each of the components. (a) Percentage of argon atoms = Number i Units (b) Percentage of neon atoms = Number Units (c) Percentage of helium atoms = Number i Units
Consider a mixture of three different gases: 1.70 g of argon (molecular mass = 39.948 g/mol), 2.90 g of neon (molecular mass = 20.180 g/mol), and 3.70 g of helium (molecular mass = 4.0026 g/mol). For this mixture, determine the percentage of the total number of atoms that corresponds to each of the components. (a) Percentage of argon atoms = Number i Units (b) Percentage of neon atoms = Number Units (c) Percentage of helium atoms = Number i Units
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter16: Temperature And The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 2OQ: A cylinder with a piston holds 0.50 m3 of oxygen at an absolute pressure of 4.0 atm. The piston is...
Related questions
Question
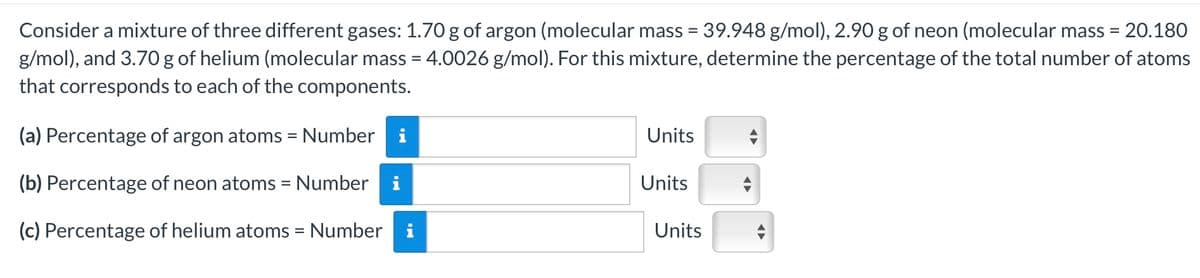
Transcribed Image Text:Consider a mixture of three different gases: 1.70 g of argon (molecular mass = 39.948 g/mol), 2.90 g of neon (molecular mass = 20.180
g/mol), and 3.70 g of helium (molecular mass = 4.0026 g/mol). For this mixture, determine the percentage of the total number of atoms
that corresponds to each of the components.
(a) Percentage of argon atoms = Number i
Units
(b) Percentage of neon atoms = Number
Units
(c) Percentage of helium atoms = Number i
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning