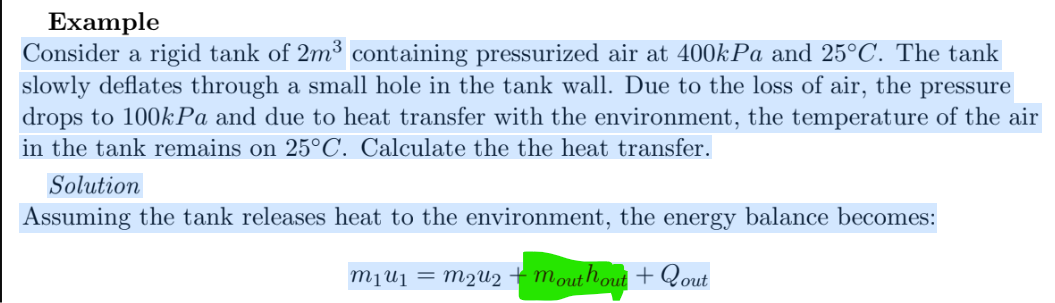
Consider a rigid tank of 2m³ containing pressurized air at 400kPa and 25°C. The tank slowly deflates through a small hole in the tank wall. Due to the loss of air, the pressure drops to 100kPa and due to heat transfer with the environment, the temperature of the air in the tank remains on 25°C. Calculate the the heat transfer. Solution Assuming the tank releases heat to the environment, the energy balance becomes: mouthout + Qout m₁u1 = m 2 U 2
Consider a rigid tank of 2m³ containing pressurized air at 400kPa and 25°C. The tank slowly deflates through a small hole in the tank wall. Due to the loss of air, the pressure drops to 100kPa and due to heat transfer with the environment, the temperature of the air in the tank remains on 25°C. Calculate the the heat transfer. Solution Assuming the tank releases heat to the environment, the energy balance becomes: mouthout + Qout m₁u1 = m 2 U 2
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter3: Transient Heat Conduction
Section: Chapter Questions
Problem 3.21P
Related questions
Question
I dont understand the green part?
Transcribed Image Text:Example
Consider a rigid tank of 2m³ containing pressurized air at 400kPa and 25°C. The tank
slowly deflates through a small hole in the tank wall. Due to the loss of air, the pressure
drops to 100kPa and due to heat transfer with the environment, the temperature of the air
in the tank remains on 25°C. Calculate the the heat transfer.
Solution
Assuming the tank releases heat to the environment, the energy balance becomes:
M₁ U₁ = M₂U₂ + mouthout + Qout
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning