Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter17: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 118CP: Consider the reaction H2(g)+Br2(g)2HBr(g) where H = 103.8 kJ/mol. In a particular experiment, equal...
Related questions
Question
Solve part 2
Transcribed Image Text:li 67%
ing 14.8
E 05/04/21
SCORE
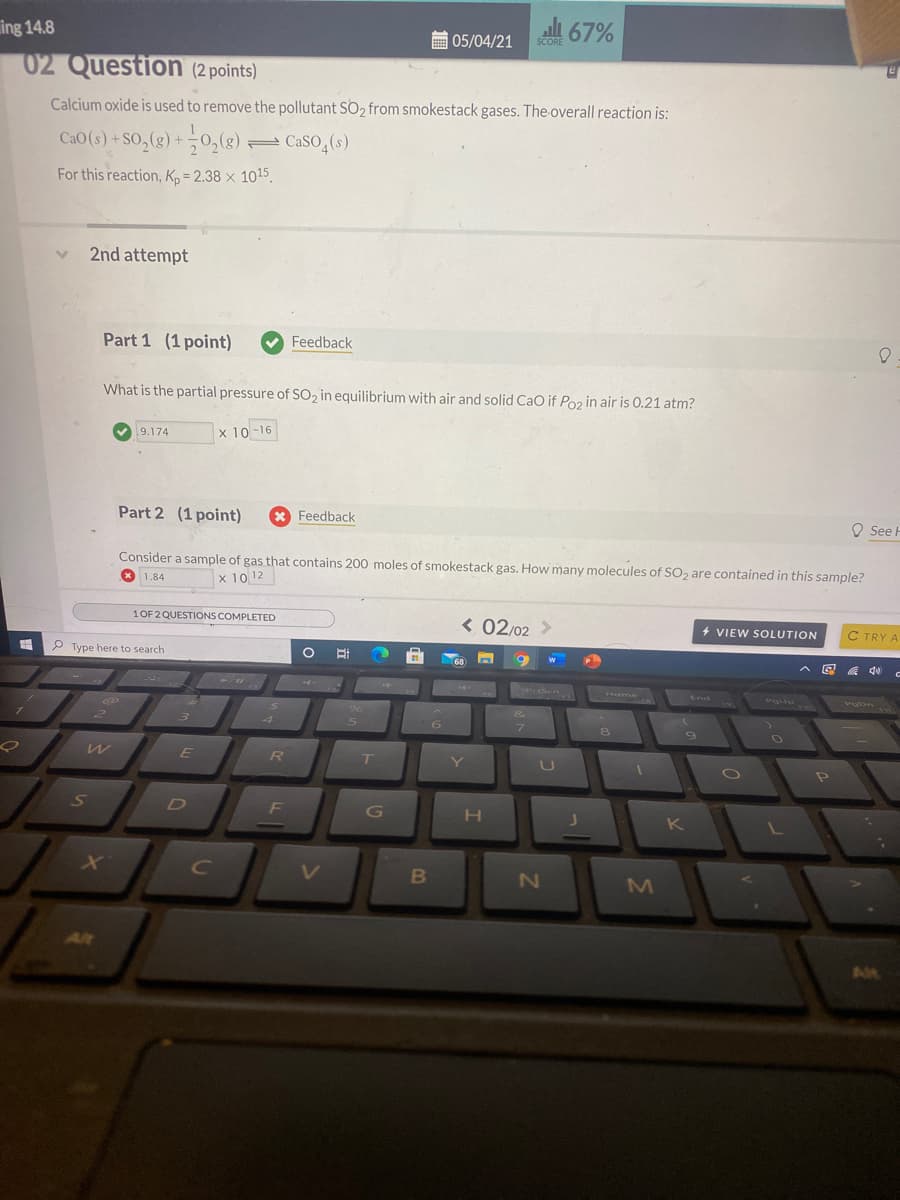
02 Question (2 points)
Calcium oxide is used to remove the pollutant SO2 from smokestack gases. The overall reaction is:
CaO (s) +SO,(g) +0,(g) CaSO,(s)
For this reaction, Kp = 2.38 × 1015.
2nd attempt
Part 1 (1 point)
Feedback
What is the partial pressure of SO2 in equilibrium with air and solid CaO if Poz in air is 0.21 atm?
9.174
x 10-16
Part 2 (1 point)
Feedback
O See F
Consider a sample of gas that contains 200 moles of smokestack gas. How many molecules of SO, are contained in this sample?
* 1.84
x 10 12
1OF 2 QUESTIONS COMPLETED
< 02/02 >
+ VIEW SOLUTION
C TRY A
P Type here to search
PiScn
Home
End
POUD
ల
vODn
6.
E
R
T
G
H
K
L
C
VT
2N
M
Alt
Alt
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax