Consider all of the following data and answer parts (A) through (C): When ketone A is treated with an equivalent of CH3Mgl in Et₂0 followed by acid (H30+) workup, two constitutional isomers B and C (C5H100) are isolated. The minor isomer B is a ketone. Isomer C decolorizes solutions of Br₂ and permagnate (i.e. it's an alkene - Chem210). When treated with HCl(aq), C gives two constitutional isomers D and E (C5H9Cl) - Chem210. Chloride D, the major product gives 2-methyl-1,3-butadiene when treated with potassium t-butoxide - Chem210. Compound B also is identical to the product obtained from the oxymercuration of 1-pentyne. (A) Identify compounds A through E by structure. (B) Classify both the A → B and A → C. (C) Write mechanisms for the A ⇒ C and C D + E reactions.
Consider all of the following data and answer parts (A) through (C): When ketone A is treated with an equivalent of CH3Mgl in Et₂0 followed by acid (H30+) workup, two constitutional isomers B and C (C5H100) are isolated. The minor isomer B is a ketone. Isomer C decolorizes solutions of Br₂ and permagnate (i.e. it's an alkene - Chem210). When treated with HCl(aq), C gives two constitutional isomers D and E (C5H9Cl) - Chem210. Chloride D, the major product gives 2-methyl-1,3-butadiene when treated with potassium t-butoxide - Chem210. Compound B also is identical to the product obtained from the oxymercuration of 1-pentyne. (A) Identify compounds A through E by structure. (B) Classify both the A → B and A → C. (C) Write mechanisms for the A ⇒ C and C D + E reactions.
Chapter19: Aldehydes And Ketones: Nucleophilic Addition Reactions
Section19.SE: Something Extra
Problem 50MP
Related questions
Question
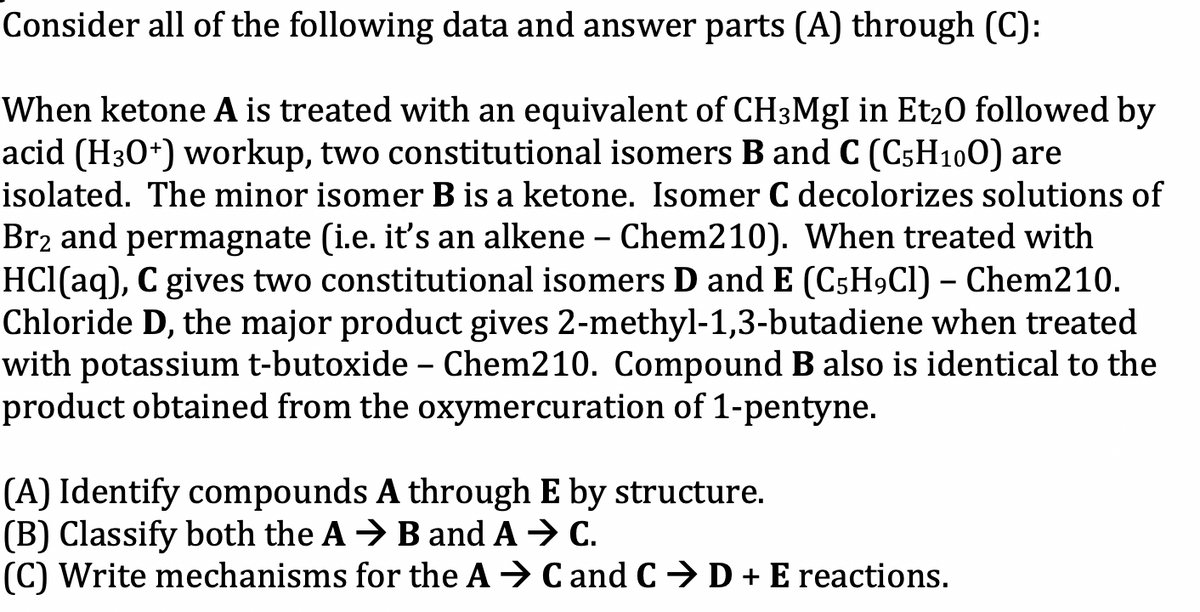
Transcribed Image Text:Consider all of the following data and answer parts (A) through (C):
When ketone A is treated with an equivalent of CH3MgI in Et₂0 followed by
acid (H30+) workup, two constitutional isomers B and C (C5H100) are
isolated. The minor isomer B is a ketone. Isomer C decolorizes solutions of
Br₂ and permagnate (i.e. it's an alkene - Chem210). When treated with
HCl(aq), C gives two constitutional isomers D and E (C5H9Cl) - Chem210.
Chloride D, the major product gives 2-methyl-1,3-butadiene when treated
with potassium t-butoxide - Chem210. Compound B also is identical to the
product obtained from the oxymercuration of 1-pentyne.
(A) Identify compounds A through E by structure.
(B) Classify both the AB and A → C.
(C) Write mechanisms for the AC and C D + E reactions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning