Consider light with a wavelength of 5.41 × 101° m. The energy of a single photon of this type of light is 3.67 x 101* J, and it takes 7.44 x 10ʻ J to heat a cup of water. How many photons would be required to heat 1.00 cup (237 g) of water?
Consider light with a wavelength of 5.41 × 101° m. The energy of a single photon of this type of light is 3.67 x 101* J, and it takes 7.44 x 10ʻ J to heat a cup of water. How many photons would be required to heat 1.00 cup (237 g) of water?
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter6: The Structure Of Atoms
Section: Chapter Questions
Problem 13PS: The most prominent line in the spectrum of mercury is at 253.652 nm. Other lines are located at...
Related questions
Question
Transcribed Image Text:101 Chem101
Get Homework Help With Cheg × b Chemistry Question | bartleby x
9 Screen Shot 2021-09-13 at 2.1 X
Screen Shot 2021-09-13 at 2.1 X +
app.101edu.co
M
Apps
G
M Gmail
YouTube
Maps
а АМAZON
Translate
Gflights
Case Status Onlin...
Reading List
Question 20.d of 30
Submit
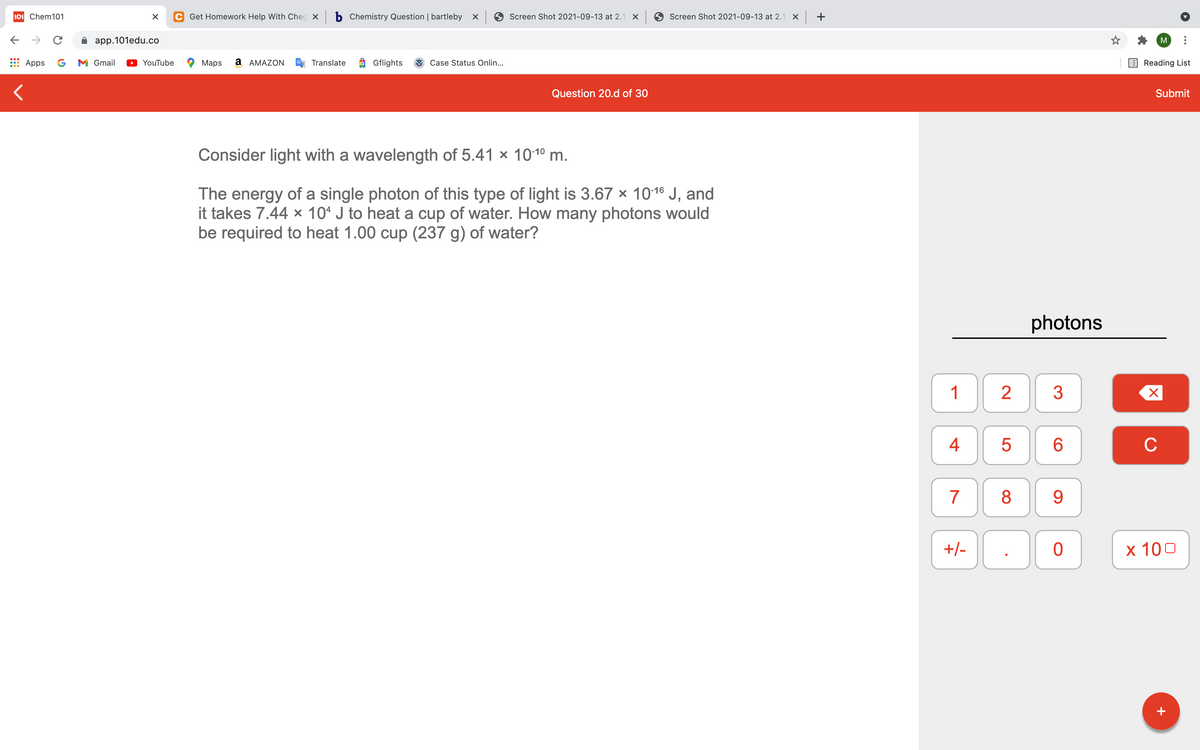
Consider light with a wavelength of 5.41 x 1010 m.
The energy of a single photon of this type of light is 3.67 x 1016 J, and
it takes 7.44 x 10* J to heat a cup of water. How many photons would
be required to heat 1.00 cup (237 g) of water?
photons
1
2
4
C
7
8
9
+/-
х 100
+
LO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning