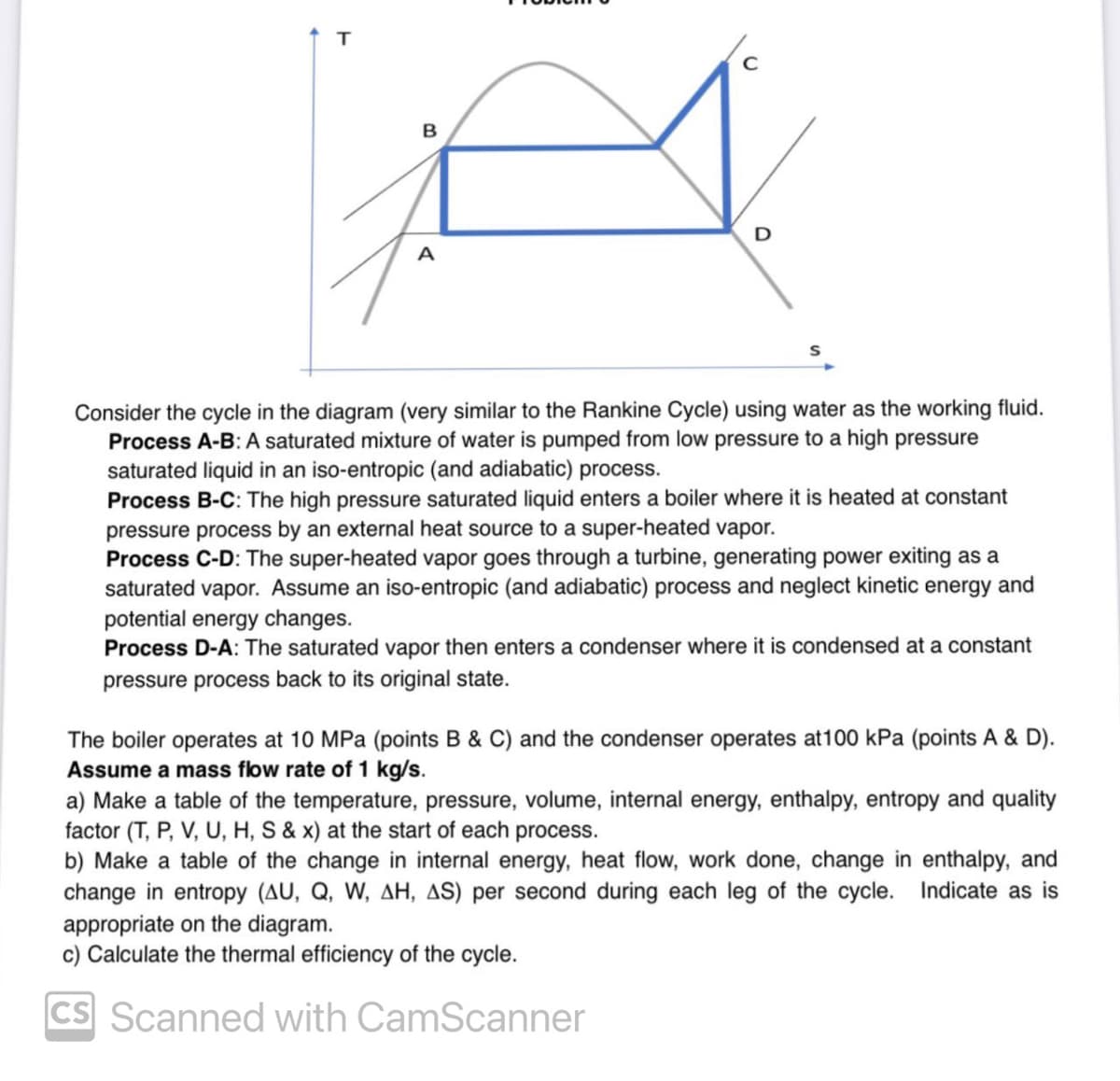
Consider the cycle in the diagram (very similar to the Rankine Cycle) using water as the working fluid. Process A-B: A saturated mixture of water is pumped from low pressure to a high pressure saturated liquid in an iso-entropic (and adiabatic) process. Process B-C: The high pressure saturated liquid enters a boiler where it is heated at constant pressure process by an external heat source to a super-heated vapor. Process C-D: The super-heated vapor goes through a turbine, generating power exiting as a saturated vapor. Assume an iso-entropic (and adiabatic) process and neglect kinetic energy and potential energy changes. Process D-A: The saturated vapor then enters a condenser where it is condensed at a constant pressure process back to its original state. The boiler operates at 10 MPa (points B & C) and the condenser operates at100 kPa (points A & D). Assume a mass flow rate of 1 kg/s. a) Make a table of the temperature, pressure, volume, internal energy, enthalpy, entropy and quality factor (T, P, V, U, H, S & x) at the start of each process. b) Make a table of the change in internal energy, heat flow, work done, change in enthalpy, and change in entropy (AU, Q, W, AH, AS) per second during each leg of the cycle. Indicate as is appropriate on the diagram. c) Calculate the thermal efficiency of the cycle. CS Scanned with CamScanner
Consider the cycle in the diagram (very similar to the Rankine Cycle) using water as the working fluid. Process A-B: A saturated mixture of water is pumped from low pressure to a high pressure saturated liquid in an iso-entropic (and adiabatic) process. Process B-C: The high pressure saturated liquid enters a boiler where it is heated at constant pressure process by an external heat source to a super-heated vapor. Process C-D: The super-heated vapor goes through a turbine, generating power exiting as a saturated vapor. Assume an iso-entropic (and adiabatic) process and neglect kinetic energy and potential energy changes. Process D-A: The saturated vapor then enters a condenser where it is condensed at a constant pressure process back to its original state. The boiler operates at 10 MPa (points B & C) and the condenser operates at100 kPa (points A & D). Assume a mass flow rate of 1 kg/s. a) Make a table of the temperature, pressure, volume, internal energy, enthalpy, entropy and quality factor (T, P, V, U, H, S & x) at the start of each process. b) Make a table of the change in internal energy, heat flow, work done, change in enthalpy, and change in entropy (AU, Q, W, AH, AS) per second during each leg of the cycle. Indicate as is appropriate on the diagram. c) Calculate the thermal efficiency of the cycle. CS Scanned with CamScanner
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
Transcribed Image Text:T
B
A
D
Consider the cycle in the diagram (very similar to the Rankine Cycle) using water as the working fluid.
Process A-B: A saturated mixture of water is pumped from low pressure to a high pressure
saturated liquid in an iso-entropic (and adiabatic) process.
Process B-C: The high pressure saturated liquid enters a boiler where it is heated at constant
pressure process by an external heat source to a super-heated vapor.
Process C-D: The super-heated vapor goes through a turbine, generating power exiting as a
saturated vapor. Assume an iso-entropic (and adiabatic) process and neglect kinetic energy and
potential energy changes.
Process D-A: The saturated vapor then enters a condenser where it is condensed at a constant
pressure process back to its original state.
The boiler operates at 10 MPa (points B & C) and the condenser operates at 100 kPa (points A & D).
Assume a mass flow rate of 1 kg/s.
a) Make a table of the temperature, pressure, volume, internal energy, enthalpy, entropy and quality
factor (T, P, V, U, H, S & x) at the start of each process.
b) Make a table of the change in internal energy, heat flow, work done, change in enthalpy, and
change in entropy (AU, Q, W, AH, AS) per second during each leg of the cycle. Indicate as is
appropriate on the diagram.
c) Calculate the thermal efficiency of the cycle.
CS Scanned with CamScanner
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 7 images

Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY