Consider the equilibrium system described by the chemical reaction below, which has a value of Kc equal to 1.2 x 10 at a certain temperature. If a solid sample of NH4SH decomposes, what will the equilibrium concentration of NH3 be? NH,SH(s) = NH3(g) + H2S(g) PREV 1 3 NEXT Based on your ICE table, set up the expression for Kc in order to determine the unknown. Do not combine or simplify terms. [1.2 x 104 - x}? -4 Ko = 1.2 x 10
Consider the equilibrium system described by the chemical reaction below, which has a value of Kc equal to 1.2 x 10 at a certain temperature. If a solid sample of NH4SH decomposes, what will the equilibrium concentration of NH3 be? NH,SH(s) = NH3(g) + H2S(g) PREV 1 3 NEXT Based on your ICE table, set up the expression for Kc in order to determine the unknown. Do not combine or simplify terms. [1.2 x 104 - x}? -4 Ko = 1.2 x 10
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 1ALQ: Consider an equilibrium mixture of four chemicals (A, B, C, and D, all gases) reacting in a closed...
Related questions
Question
![Consider the equilibrium system described by the chemical reaction below, which has a
value of Kc equal to 1.2 × 10 at a certain temperature. If a solid sample of NH4SH
decomposes, what will the equilibrium concentration of NH3 be?
NH,SH(s) = NH3(g) + H2S(g)
PREV
1
NEXT
Based on your ICE table, set up the expression for Kc in order to determine the unknown. Do not combine or
simplify terms.
[1.2 x 104 - x]?
Ko
= 1.2 x 10-4
%3D
[2x]
5 RESET
[2x]?
[1.2 x 104 + x]
[1.2 x 104 - x]
(1.2 x 10 + 2x] (1.2 x 104 - 2x]
[1.2 x 10 + x)
[x]
[2x]
[1.2 x 104 - x²
[1.2 x 104 + 2xP
[1.2 x 10* - 2x}](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Faeef920a-b7fc-42c4-a405-16f92aebf3b1%2Faa182067-badb-4100-82ff-5550f1a7adc8%2Fgl4p0u_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Consider the equilibrium system described by the chemical reaction below, which has a
value of Kc equal to 1.2 × 10 at a certain temperature. If a solid sample of NH4SH
decomposes, what will the equilibrium concentration of NH3 be?
NH,SH(s) = NH3(g) + H2S(g)
PREV
1
NEXT
Based on your ICE table, set up the expression for Kc in order to determine the unknown. Do not combine or
simplify terms.
[1.2 x 104 - x]?
Ko
= 1.2 x 10-4
%3D
[2x]
5 RESET
[2x]?
[1.2 x 104 + x]
[1.2 x 104 - x]
(1.2 x 10 + 2x] (1.2 x 104 - 2x]
[1.2 x 10 + x)
[x]
[2x]
[1.2 x 104 - x²
[1.2 x 104 + 2xP
[1.2 x 10* - 2x}
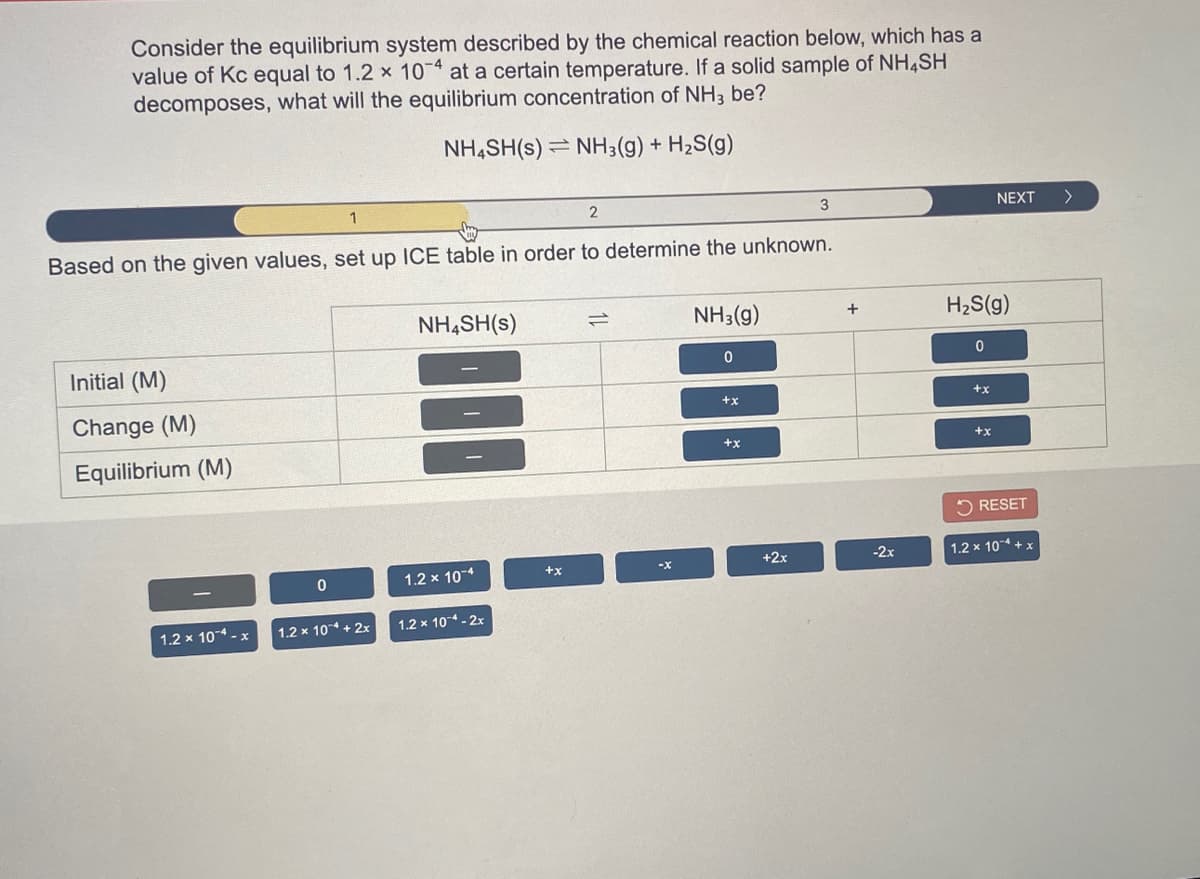
Transcribed Image Text:Consider the equilibrium system described by the chemical reaction below, which has a
value of Kc equal to 1.2 x 10 at a certain temperature. If a solid sample of NH4SH
decomposes, what will the equilibrium concentration of NH3 be?
NH,SH(s) = NH3(g) +
H2S(g)
1
2
3
NEXT
>
Based on the given values, set up ICE table in order to determine the unknown.
NH,SH(s)
NH3(g)
H2S(g)
+
Initial (M)
++
+x
Change (M)
++
+x
Equilibrium (M)
5 RESET
-2x
1.2 x 104 + x
+2x
+x
1.2 x 10-4
1.2 x 10 + 2x
1.2 x 10 - 2x
1.2 x 104 - x
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning