Consider the equilibrium system described by the chemical reaction below, which has a value of Kc equal to 0.042 at a certain temperature. If 2.860 g of PCIS initially decomposes in a 700 mL closed container, what will the equilibrium concentration of PCIs be? PCI:(g) PCL(g) + CL(g) PREV NEXT Based on your ICE table, set up the expression for Kc in order to determine the unknown. Do not combine or implify terms. [2.860 + x] = 0.042 [4.086 - 2x] O RESET [x] [2x] [2x] [2.860 + x] [2.860 - x] [2.860 - 2x] [0.01374 + x] [0.01374 - x) [0.01374 - 2x] [0.01982 + x] [0.01962 - 1] [0.01002 - 2x) [4.086 + x] [4.086 - x] [4.086 - 2x]
Consider the equilibrium system described by the chemical reaction below, which has a value of Kc equal to 0.042 at a certain temperature. If 2.860 g of PCIS initially decomposes in a 700 mL closed container, what will the equilibrium concentration of PCIs be? PCI:(g) PCL(g) + CL(g) PREV NEXT Based on your ICE table, set up the expression for Kc in order to determine the unknown. Do not combine or implify terms. [2.860 + x] = 0.042 [4.086 - 2x] O RESET [x] [2x] [2x] [2.860 + x] [2.860 - x] [2.860 - 2x] [0.01374 + x] [0.01374 - x) [0.01374 - 2x] [0.01982 + x] [0.01962 - 1] [0.01002 - 2x) [4.086 + x] [4.086 - x] [4.086 - 2x]
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter15: Principles Of Chemical Reactivity: Equilibria
Section: Chapter Questions
Problem 40GQ: Consider the following equilibrium: COBr2(g) CO(g) + Br2(g)Kc = 0.190 at 73 C (a) A 0.50 mol sample...
Related questions
Question
100%
![Question 42 of 44
Consider the equilibrium system described by the chemical reaction below, which has a value of
Kc equal to 0.042 at a certain temperature. If 2.860 g of PCIs initially decomposes in a 700 mL
closed container, what will the equilibrium concentration of PCIs be?
PCL(g) PCL(g) + CL(g)
PREV
2
NEXT
Based on your ICE table, set up the expression for Kc in order to determine the unknown. Do not combine or
simplify terms.
[2.860 + x]
Kc =
= 0.042
[4.086 - 2x]
O RESET
[x]
[2x]
[2.860 + x]
[2.860 - x]
[2.860 - 21]
[0.01374 + x]
[0.01374 - x]
[0.01374 - 2x]
[0.01962 + x]
0.01962 - 1]
[0.01062 - 2x]
[4.086 + z]
[4.086 - x]
[4.086 - 2x]](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fd862c25f-a636-4347-831e-84b629db5903%2Fc76dbe38-50a3-464a-aaf8-3ab1db92b49a%2Fh68p4qe_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Question 42 of 44
Consider the equilibrium system described by the chemical reaction below, which has a value of
Kc equal to 0.042 at a certain temperature. If 2.860 g of PCIs initially decomposes in a 700 mL
closed container, what will the equilibrium concentration of PCIs be?
PCL(g) PCL(g) + CL(g)
PREV
2
NEXT
Based on your ICE table, set up the expression for Kc in order to determine the unknown. Do not combine or
simplify terms.
[2.860 + x]
Kc =
= 0.042
[4.086 - 2x]
O RESET
[x]
[2x]
[2.860 + x]
[2.860 - x]
[2.860 - 21]
[0.01374 + x]
[0.01374 - x]
[0.01374 - 2x]
[0.01962 + x]
0.01962 - 1]
[0.01062 - 2x]
[4.086 + z]
[4.086 - x]
[4.086 - 2x]
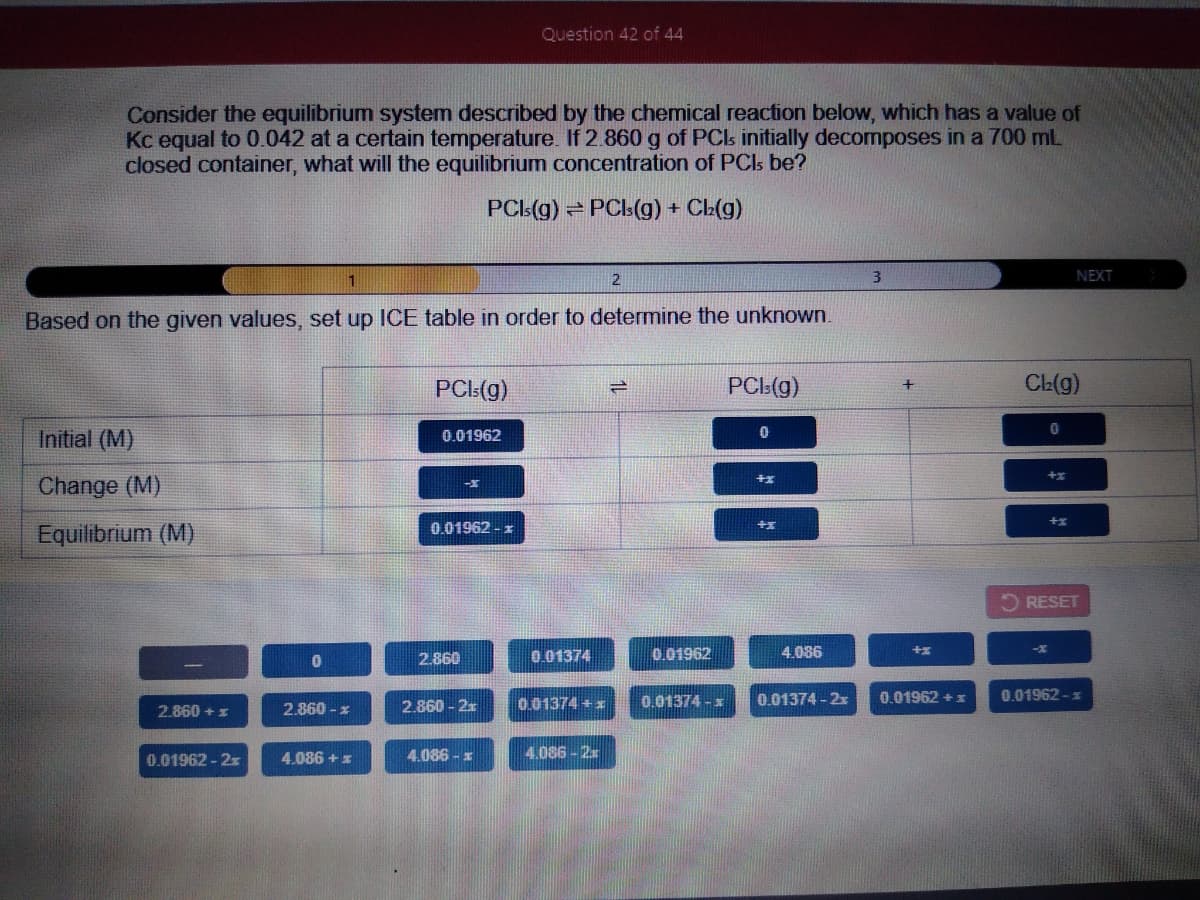
Transcribed Image Text:Question 42 of 44
Consider the equilibrium system described by the chemical reaction below, which has a value of
Kc equal to 0.042 at a certain temperature. If 2.860 g of PCs initially decomposes in a 700 ml
closed container, what will the equilibrium concentration of PCIs be?
PCL(g) PCls(g) +
CL(g)
2
3
NEXT
Based on the given values, set up ICE table in order to determine the unknown.
PCL(g)
PCI:(g)
CL(g)
Initial (M)
0.01962
Change (M)
0.01962 - z
Equilibrium (M)
2 RESET
2.860
0.01374
0.01962
4.086
0.01374 + x
0.01374 -x
0.01374 - 2x
0.01962 +:
0.01962 -x
2.860 + x
2.860 - x
2.860 - 2x
0.01962-2x
4.086 + z
4.086 - x
4.086-2x
Expert Solution
Step 1
Kc denotes the ratio of concentration of the products to the reactants raise to the power of their stoichiometric coefficients when the reaction is in equilibrium.
Kc is a function of temperature depending upon the nature of reaction.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning