Consider the following acidic equilibrium: H:CO:(aq) + H20(1) = HCO: (aq) + H:O (aq). If you add NaHCOs to this solution, which of the following will occur? A) The reaction quotient will decrease. B) The reaction will shift in the reverse direction. C) The equilibrium constant will increase. D) No changes to the equilibrium positions will take place.
Consider the following acidic equilibrium: H:CO:(aq) + H20(1) = HCO: (aq) + H:O (aq). If you add NaHCOs to this solution, which of the following will occur? A) The reaction quotient will decrease. B) The reaction will shift in the reverse direction. C) The equilibrium constant will increase. D) No changes to the equilibrium positions will take place.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter16: Acid-base Equilibria
Section: Chapter Questions
Problem 16.112QP: Ionization of the first proton from H2SeO4 is complete (H2SeO4 is a strong acid); the...
Related questions
Question
Solve question 19
Transcribed Image Text:Question 19 of 25
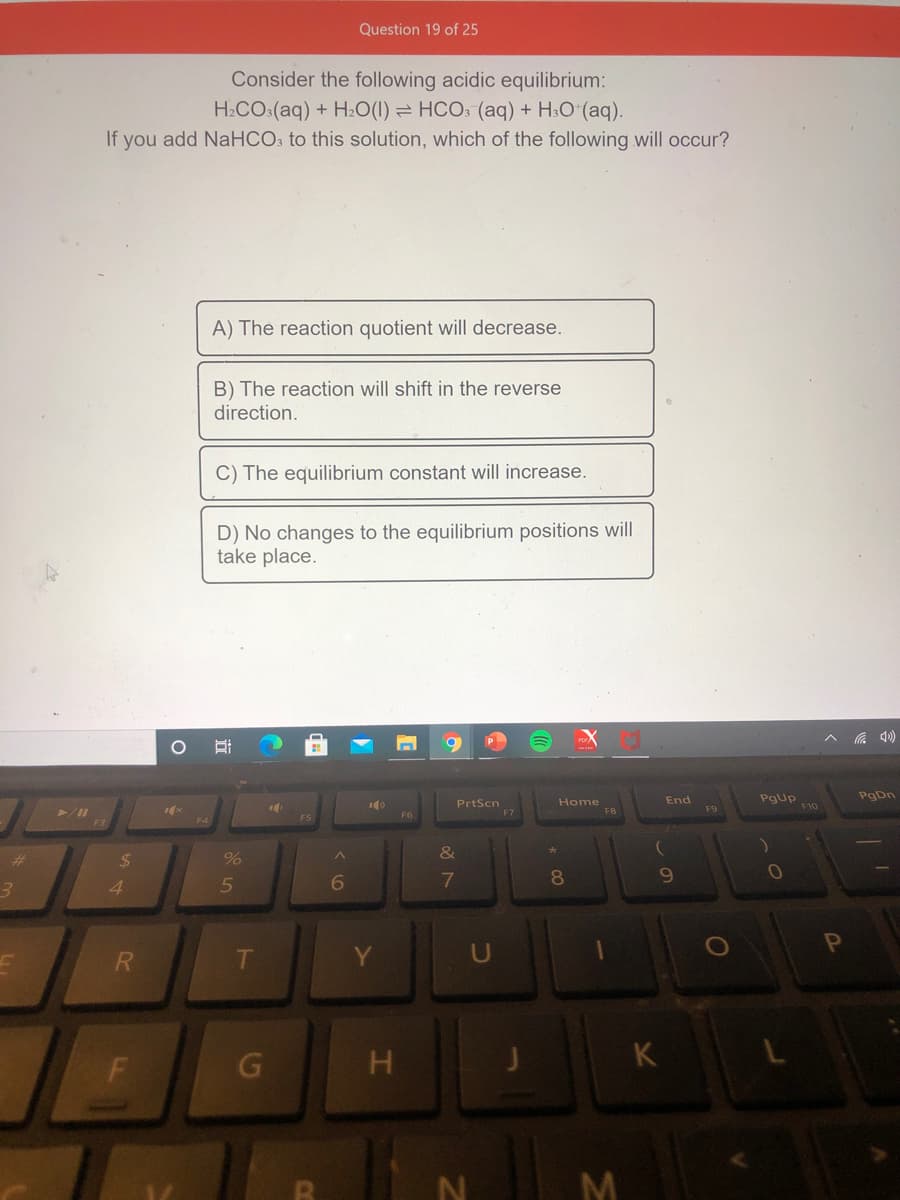
Consider the following acidic equilibrium:
H2CO:(aq) + H20(1) = HCO: (aq) + H:O (aq).
If you add NaHCO: to this solution, which of the following will occur?
A) The reaction quotient will decrease.
B) The reaction will shift in the reverse
direction.
C) The equilibrium constant will increase.
D) No changes to the equilibrium positions will
take place.
ヘ )
PgDn
PrtScn
F7
Home
FB
End
PgUp
F10
F9
F6
%23
%24
%
&
8.
10
4.
5
OP
H
K
N
M
L.
F.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning