Consider the following aqueous solutions: (i) 0.20 m HOCH2 CH2OH (nonvolatile, nonelectrolyte); (ii) 0.10 m SrI2; (iii) 0.12 m Nal; and (iv) 0.12 m Rb₂SO4. a. Which solution has the highest boiling point? O0.12 m NaI O0.20 m HOCH2 CH2OH O0.12 m Rb2SO4 O0.10 m SrI b. Which solution has the lowest freezing point? ○ 0.12 m NaI O0.20 m HOCH2 CH2OH O0.12 m Rb2SO4 00.10 m SrI₂ c. Which solution has the highest water vapor pressure? O0.12 m NaI O0.20 m HOCH₂ CH₂ OH O0.12 m Rb2SO4 00.10 m SrI₂ For the gas phase decomposition of trichloroethane, CH3 CC13 CH2=CC12+ HCI the rate constant in s¹ has been determined at several temperatures. When Ink is plotted against the reciprocal of the Kelvin temperature, the resulting linear plot has a slope of -2.41 x 104 K and a y-intercept of 28.8. The value of the rate constant for the gas phase decomposition of trichloroethane at 618 K is 8 (Enter your answer to one significant figure.)
Consider the following aqueous solutions: (i) 0.20 m HOCH2 CH2OH (nonvolatile, nonelectrolyte); (ii) 0.10 m SrI2; (iii) 0.12 m Nal; and (iv) 0.12 m Rb₂SO4. a. Which solution has the highest boiling point? O0.12 m NaI O0.20 m HOCH2 CH2OH O0.12 m Rb2SO4 O0.10 m SrI b. Which solution has the lowest freezing point? ○ 0.12 m NaI O0.20 m HOCH2 CH2OH O0.12 m Rb2SO4 00.10 m SrI₂ c. Which solution has the highest water vapor pressure? O0.12 m NaI O0.20 m HOCH₂ CH₂ OH O0.12 m Rb2SO4 00.10 m SrI₂ For the gas phase decomposition of trichloroethane, CH3 CC13 CH2=CC12+ HCI the rate constant in s¹ has been determined at several temperatures. When Ink is plotted against the reciprocal of the Kelvin temperature, the resulting linear plot has a slope of -2.41 x 104 K and a y-intercept of 28.8. The value of the rate constant for the gas phase decomposition of trichloroethane at 618 K is 8 (Enter your answer to one significant figure.)
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section: Chapter Questions
Problem 7PS
Related questions
Question
100%

Transcribed Image Text:Consider the following aqueous solutions: (i) 0.20 m HOCH2 CH2OH
(nonvolatile, nonelectrolyte); (ii) 0.10 m SrI2; (iii) 0.12 m Nal; and (iv) 0.12 m
Rb₂SO4.
a. Which solution has the highest boiling point?
O0.12 m NaI
O0.20 m HOCH2 CH2OH
O0.12 m Rb2SO4
O0.10 m SrI
b. Which solution has the lowest freezing point?
○ 0.12 m NaI
O0.20 m HOCH2 CH2OH
O0.12 m Rb2SO4
00.10 m SrI₂
c. Which solution has the highest water vapor pressure?
O0.12 m NaI
O0.20 m HOCH₂ CH₂ OH
O0.12 m Rb2SO4
00.10 m SrI₂
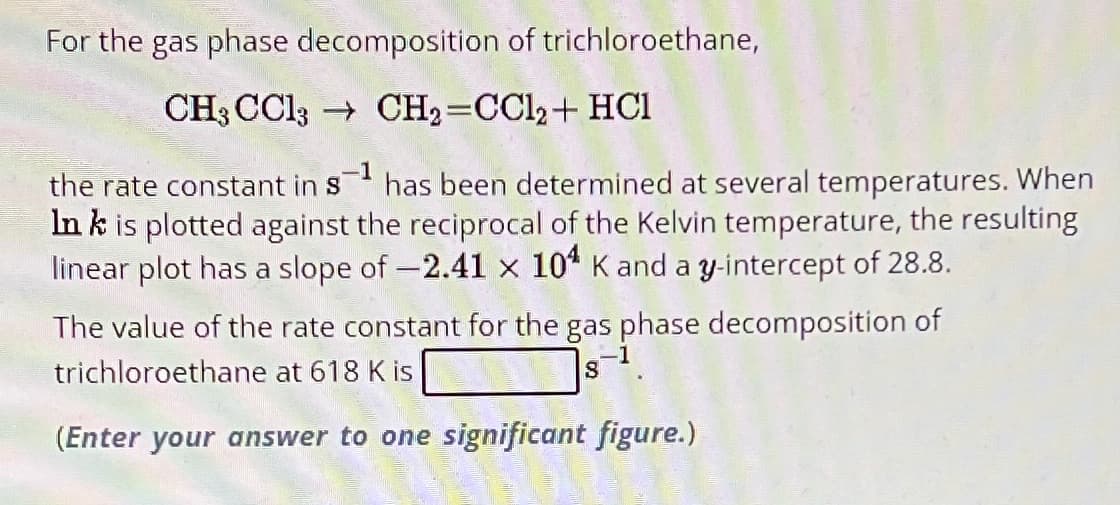
Transcribed Image Text:For the gas phase decomposition of trichloroethane,
CH3 CC13
CH2=CC12+ HCI
the rate constant in s¹ has been determined at several temperatures. When
Ink is plotted against the reciprocal of the Kelvin temperature, the resulting
linear plot has a slope of -2.41 x 104 K and a y-intercept of 28.8.
The value of the rate constant for the gas phase decomposition of
trichloroethane at 618 K is
8
(Enter your answer to one significant figure.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning