Consider the following chemical reaction at equilibrium: HF (aq) + H₂O(l) = H³O+(aq) + F¯(aq) If one drop of aqueous hydrochloric acid (HCI) is added, how will Keq for the reaction change? A) increase B) decrease C) stay the same
Consider the following chemical reaction at equilibrium: HF (aq) + H₂O(l) = H³O+(aq) + F¯(aq) If one drop of aqueous hydrochloric acid (HCI) is added, how will Keq for the reaction change? A) increase B) decrease C) stay the same
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.92PAE: Consider the following system: 4NH3(g)+3O2(g)2N2(g)+6H2O(l)H=153.04kJ (a) How will the amount of...
Related questions
Question
Provide explanation of correct and incorrect option. please do not provide solution based on AI.
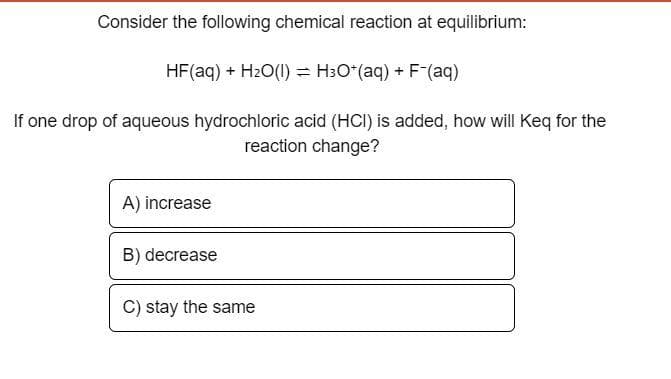
Transcribed Image Text:Consider the following chemical reaction at equilibrium:
HF (aq) + H₂O(l) = H³O+(aq) + F¯(aq)
If one drop of aqueous hydrochloric acid (HCI) is added, how will Keq for the
reaction change?
A) increase
B) decrease
C) stay the same
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
