Consider the following data for bromine: g mol atomic mass electronegativity ionization energy 79.904- electron affinity 324.6 heat of fusion 2.96 1139.9 5.8 kJ mol kJ mol kJ mol You may find additional useful data in the ALEKS Data tab. Does the following reaction absorb or release energy? (1) Br (g) + e → Br(g) Is it possible to calculate the amount of energy absorbed or released by reaction (1) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (1): release absorb O Can't be decided with the data given. yes O no kJ/mol E 0 C
Consider the following data for bromine: g mol atomic mass electronegativity ionization energy 79.904- electron affinity 324.6 heat of fusion 2.96 1139.9 5.8 kJ mol kJ mol kJ mol You may find additional useful data in the ALEKS Data tab. Does the following reaction absorb or release energy? (1) Br (g) + e → Br(g) Is it possible to calculate the amount of energy absorbed or released by reaction (1) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (1): release absorb O Can't be decided with the data given. yes O no kJ/mol E 0 C
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter8: Electron Configurations And Periodicity
Section: Chapter Questions
Problem 8.40QP
Related questions
Question
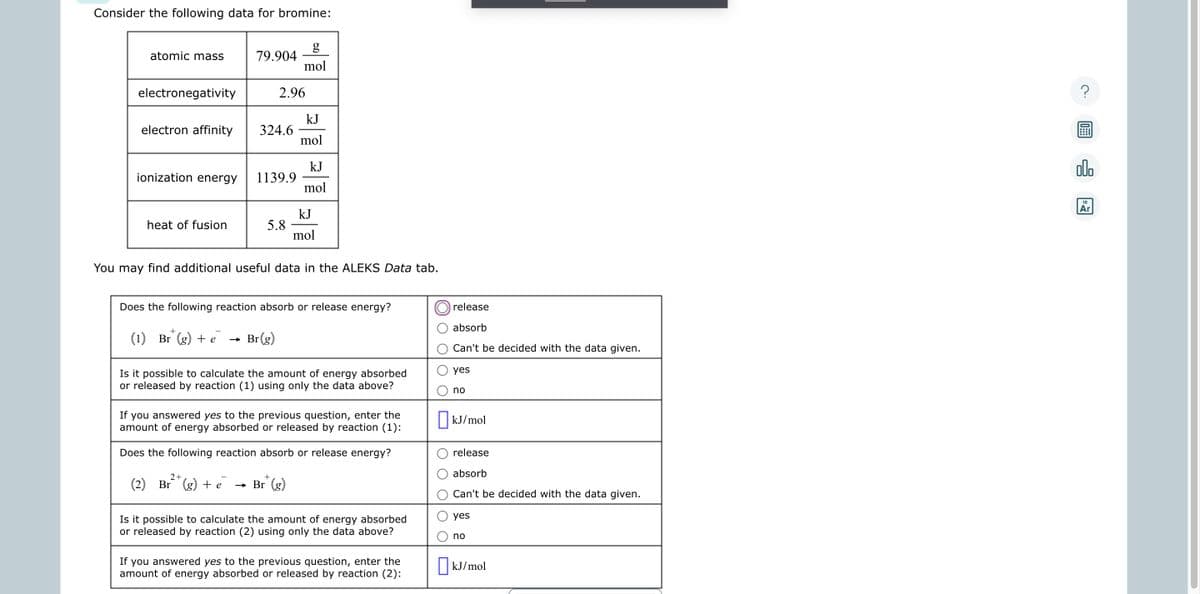
Transcribed Image Text:Consider the following data for bromine:
atomic mass
electronegativity
electron affinity
ionization energy
heat of fusion
79.904
2.96
324.6
1139.9
5.8
g
mol
kJ
mol
kJ
mol
kJ
mol
You may find additional useful data in the ALEKS Data tab.
2+
+
(2) Br (g) + e → Br (g)
Does the following reaction absorb or release energy?
(1) Br (g) + e → Br(g)
Is it possible to calculate the amount of energy absorbed
or released by reaction (1) using only the data above?
If you answered yes to the previous question, enter the
amount of energy absorbed or released by reaction (1):
Does the following reaction absorb or release energy?
Is it possible to calculate the amount of energy absorbed
or released by reaction (2) using only the data above?
If you answered yes to the previous question, enter the
amount of energy absorbed or released by reaction (2):
release
absorb
Can't be decided with the data given.
yes
no
kJ/mol
release
absorb
Can't be decided with the data given.
yes
no
kJ/mol
olo
18
Ar
Transcribed Image Text:Chrome File Edit View History
000
← → C
O
0
kJ/mol
Mail - Iz X
Explanation
OCT
31
Electronic Structure and Chemical Bonding
Deducing valence electron configuration from trends in successive...
Bookmarks Profiles Tab Window Help
Inbox (4 x
Check
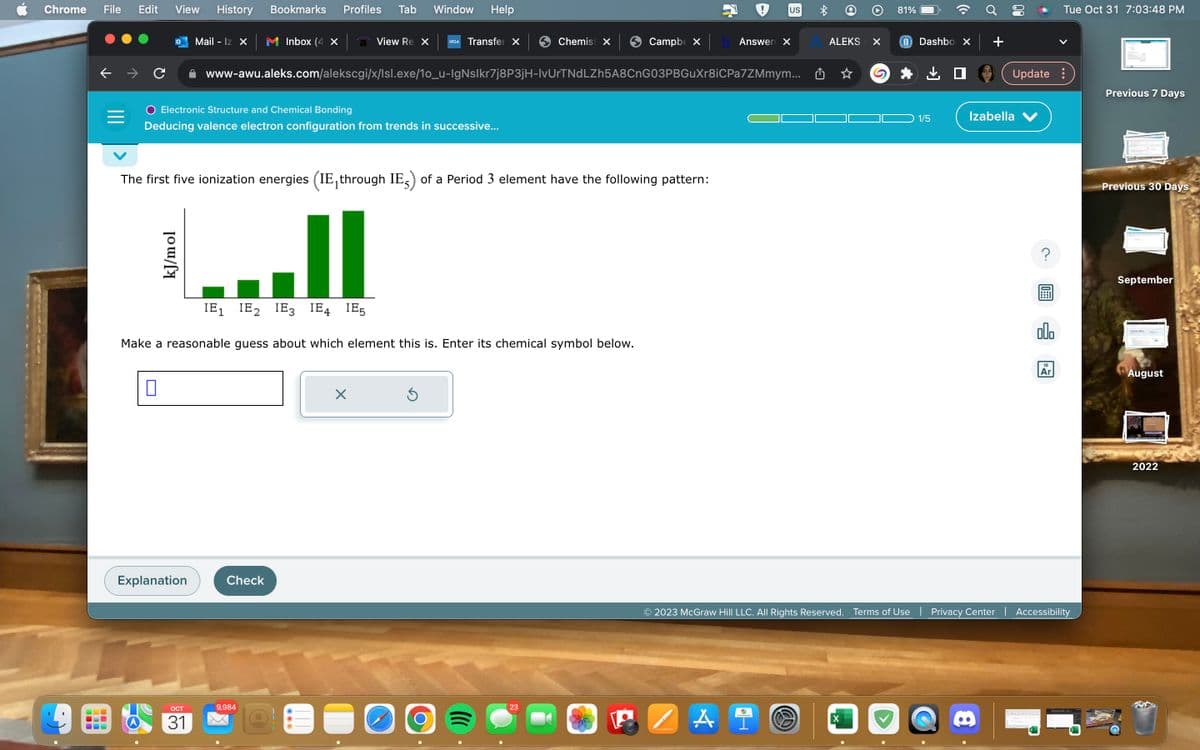
The first five ionization energies (IE, through IE) of a Period 3 element have the following pattern:
||
IE₁ IE₂ IE3 IE4 IE5
Make a reasonable guess about which element this is. Enter its chemical symbol below.
9,984
View Re X UCLA
Transfer X
X
www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-lvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmym...
Ś
Chemist X
Campbe X
23
Answer X
US
A i
40
C
ALEKS
×
X
81%
Dashbo X
1/5
Update:
Izabella V
00.
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
Tue Oct 31 7:03:48 PM
18
Ar
Previous 7 Days
Previous 30 Days
September
August
2022
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning