Consider the following data on some weak acids and weak bases: acid base Ka K, name formula name formula HCN 4.9 × 10¯10 C,H;NH, 6.4 × 10 -4 hydrocyanic acid ethylamine HNO, 4.5 x 10™ hydroxylamine HONH, |1.1 × 10~8 nitrous acid Use this data to rank the following solutions in order of increasing pH. In other words, select a 'l' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution pH 0.1 M Nal choose one a 0.1 M KCN choose one e 0.1 M NaNO2 choose one e 0.1 M C2H5NH3CI choose one a
Consider the following data on some weak acids and weak bases: acid base Ka K, name formula name formula HCN 4.9 × 10¯10 C,H;NH, 6.4 × 10 -4 hydrocyanic acid ethylamine HNO, 4.5 x 10™ hydroxylamine HONH, |1.1 × 10~8 nitrous acid Use this data to rank the following solutions in order of increasing pH. In other words, select a 'l' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution pH 0.1 M Nal choose one a 0.1 M KCN choose one e 0.1 M NaNO2 choose one e 0.1 M C2H5NH3CI choose one a
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter15: Solutions Of Acids And Bases
Section: Chapter Questions
Problem 15.114QE
Related questions
Question
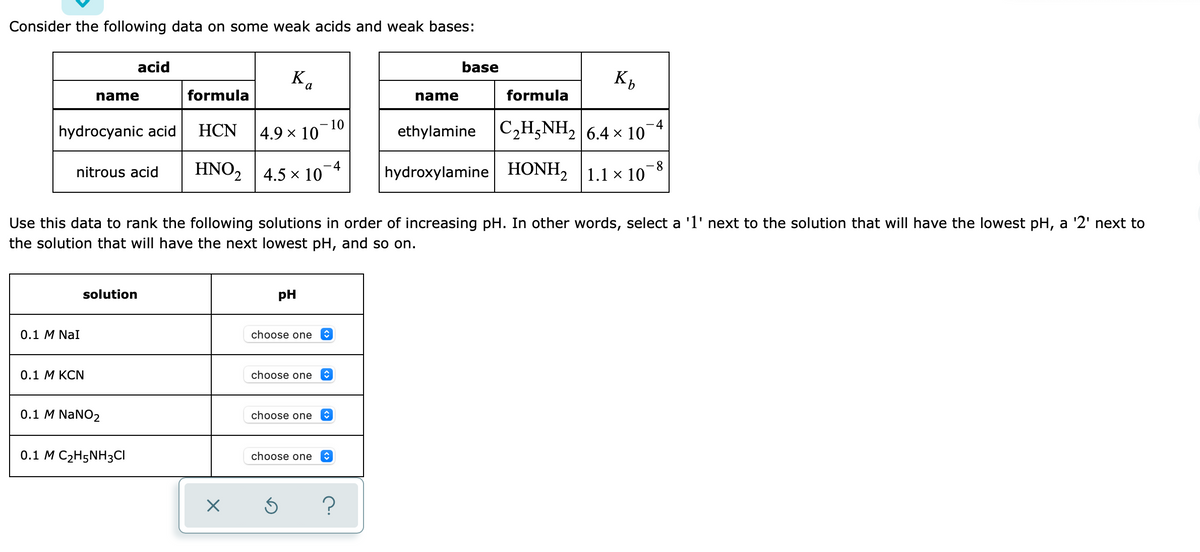
Transcribed Image Text:Consider the following data on some weak acids and weak bases:
acid
base
Ka
name
formula
name
formula
hydrocyanic acid
НCN
10
4.9 x 10
ethylamine C2H;NH, 6.4 × 10¬4
HNO2 |4.5 x 10
hydroxylamine HONH, 1.1 × 10-8
nitrous acid
Use this data to rank the following solutions in order of increasing pH. In other words, select a 'l' next to the solution that will have the lowest pH, a '2' next to
the solution that will have the next lowest pH, and so on.
solution
pH
0.1 M NaI
choose one
0.1 M KCN
choose one
0.1 M NaNO2
choose one
0.1 M C2H5NH3CI
choose one
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning