Consider the following data set: Sample 100 μg/mL CuSO4 standard 200 µg/mL CuSO4 standard 300 µg/mL CuSO4 standard 400 μg/mL CuSO4 standard 500 µg/mL CuSO4 standard CuSO4 Solution with Unknown Concentration Corrected Absorbance at 470 nm 0.032 0.058 0.090 0.114 0.140 0.070 1. Plot (Using MS Excel) or draw by hand a standard calibration curve for samples 1 to 5. Determine the molar absorptivity using the absorbance of the 100 µg/mL CuSO4 standard. (Note: Convert ug/mL to Molarity first using the molar mass of the standard which is 249.685 g/mol).
Consider the following data set: Sample 100 μg/mL CuSO4 standard 200 µg/mL CuSO4 standard 300 µg/mL CuSO4 standard 400 μg/mL CuSO4 standard 500 µg/mL CuSO4 standard CuSO4 Solution with Unknown Concentration Corrected Absorbance at 470 nm 0.032 0.058 0.090 0.114 0.140 0.070 1. Plot (Using MS Excel) or draw by hand a standard calibration curve for samples 1 to 5. Determine the molar absorptivity using the absorbance of the 100 µg/mL CuSO4 standard. (Note: Convert ug/mL to Molarity first using the molar mass of the standard which is 249.685 g/mol).
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section: Chapter Questions
Problem 77PS
Related questions
Question
Provide full complete solutions do not round off numbers and show proper labelling and cancellations
Answer question #1, use ms excel for plot and include the screenshot and.
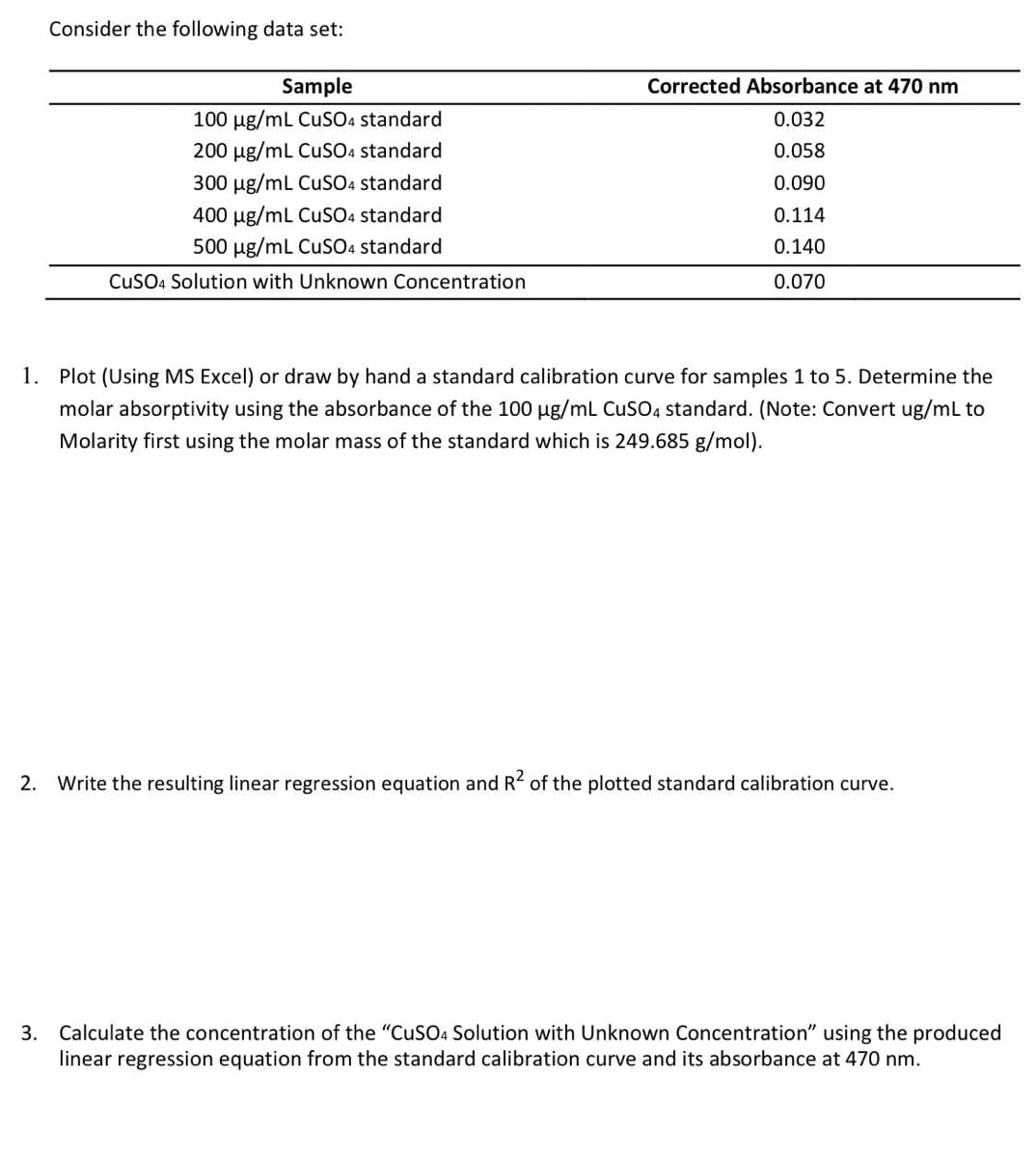
Transcribed Image Text:Consider the following data set:
Sample
100 μg/mL CuSO4 standard
200 μg/mL CuSO4 standard
300 μg/mL CuSO4 standard
400 µg/mL CuSO4 standard
500 μg/mL CuSO4 standard
CuSO4 Solution with Unknown Concentration
Corrected Absorbance at 470 nm
0.032
0.058
0.090
0.114
0.140
0.070
1. Plot (Using MS Excel) or draw by hand a standard calibration curve for samples 1 to 5. Determine the
molar absorptivity using the absorbance of the 100 µg/mL CuSO4 standard. (Note: Convert ug/mL to
Molarity first using the molar mass of the standard which is 249.685 g/mol).
2. Write the resulting linear regression equation and R² of the plotted standard calibration curve.
3. Calculate the concentration of the "CuSO4 Solution with Unknown Concentration" using the produced
linear regression equation from the standard calibration curve and its absorbance at 470 nm.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning