Consider the following half-reactions: Half-reaction E° (V) L(s) + 2e → 2I°(aq) 0.535V Co2*(aq) + 2e Co(s) -0.280V Al (aq) + 3e → Al(s)-1.660V (1) The strongest oxidizing agent is: enter formula (2) The weakest oxidizing agent is: (3) The weakest reducing agent is: (4) The strongest reducing agent is:
Consider the following half-reactions: Half-reaction E° (V) L(s) + 2e → 2I°(aq) 0.535V Co2*(aq) + 2e Co(s) -0.280V Al (aq) + 3e → Al(s)-1.660V (1) The strongest oxidizing agent is: enter formula (2) The weakest oxidizing agent is: (3) The weakest reducing agent is: (4) The strongest reducing agent is:
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 93QAP: Atomic masses can be determined by electrolysis. In one hour, a current of 0.600 A deposits 2.42 g...
Related questions
Question
Transcribed Image Text:m/ilm/takeAssignment/takeCovalentActivity.do?locator=assignment-take
Use the References to access important values if needed for this
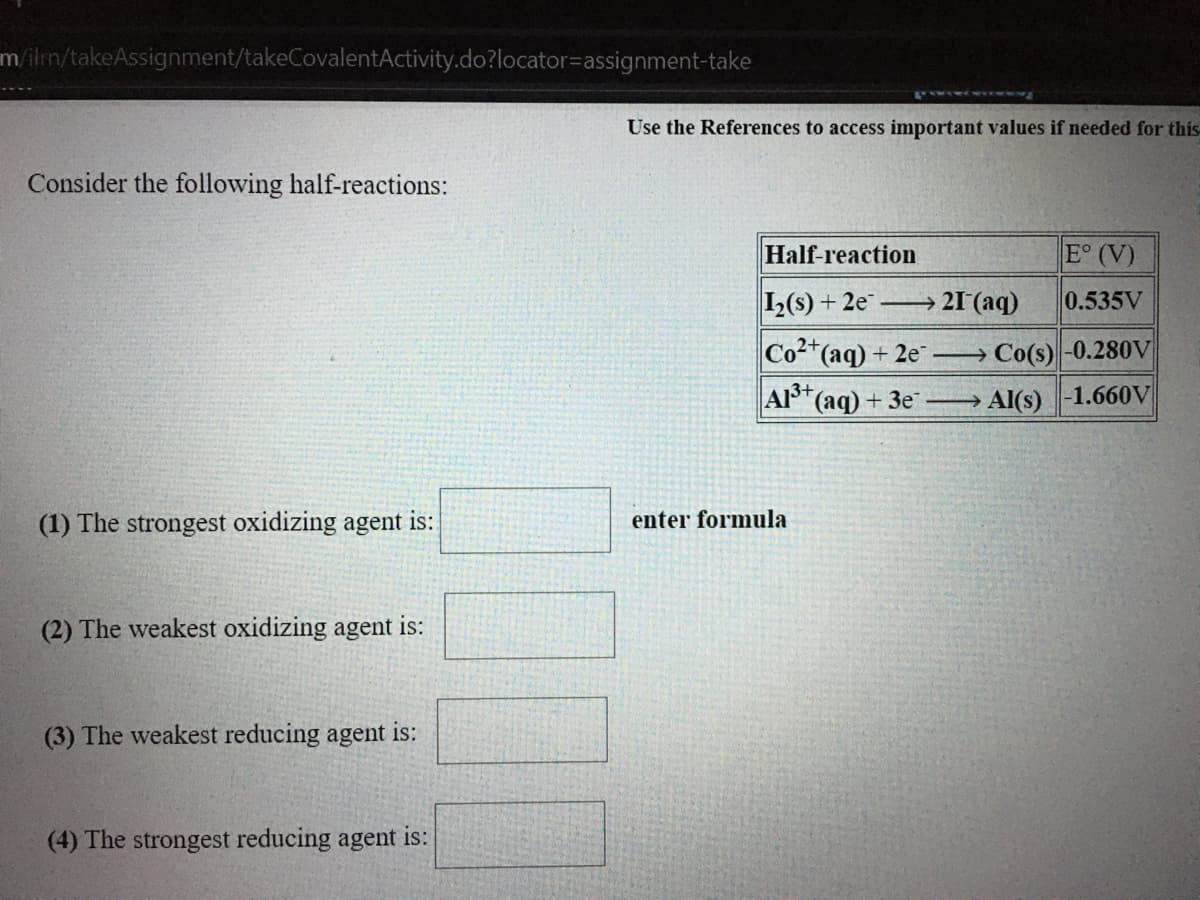
Consider the following half-reactions:
Half-reaction
E° (V)
L(s) + 2e → 21 (aq)
0.535V
Co(aq) + 2e Co(s)-0.280V
A3+
(ag) + 3e →
Al(s)-1.660V
(1) The strongest oxidizing agent is:
enter formula
(2) The weakest oxidizing agent is:
(3) The weakest reducing agent is:
(4) The strongest reducing agent is:
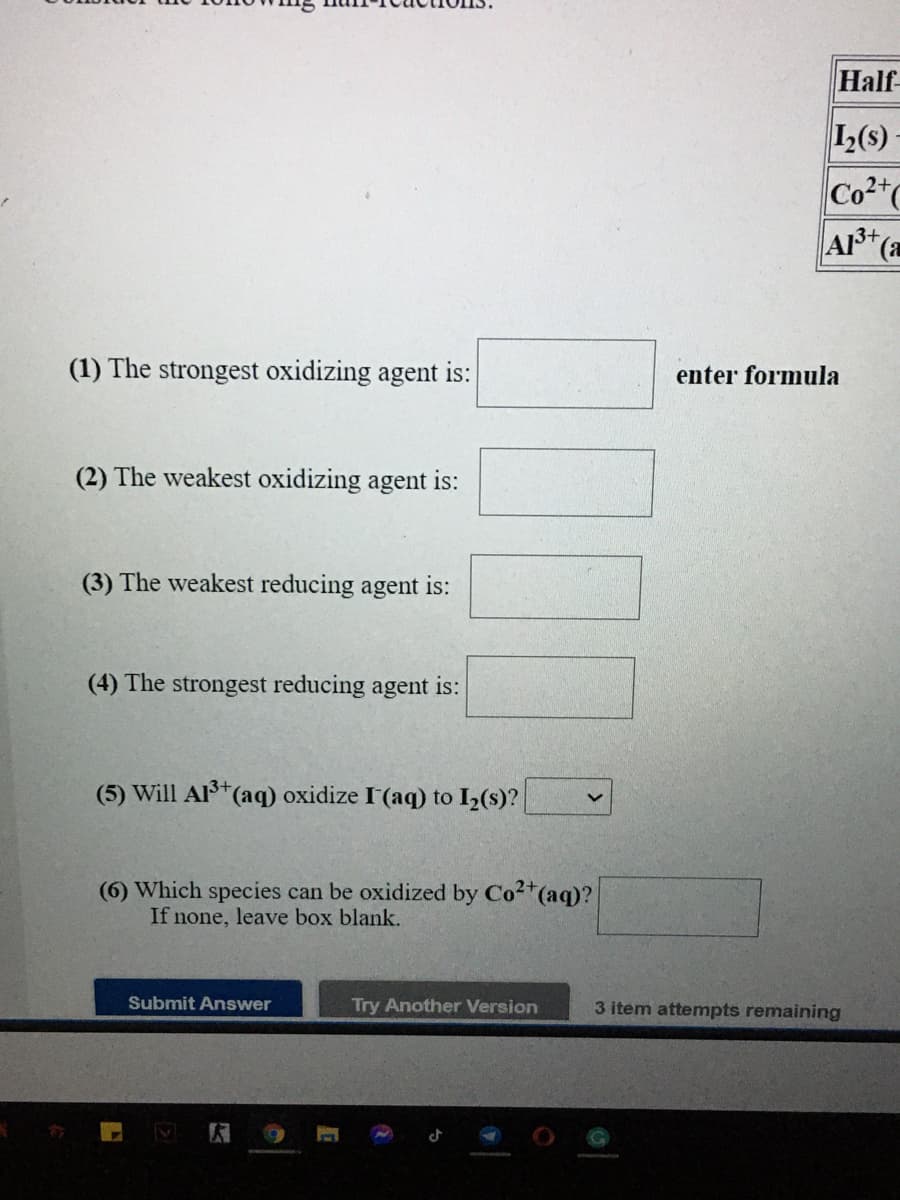
Transcribed Image Text:Half
1(s)
Co2"
A+(a
(1) The strongest oxidizing agent is:
enter formula
(2) The weakest oxidizing agent is:
(3) The weakest reducing agent is:
(4) The strongest reducing agent is:
(5) Will Al (aq) oxidize I'(aq) to I2(s)?
3+
(6) Which species can be oxidized by Co2*(aq)?
If none, leave box blank.
Submit Answer
Try Another Version
3 item attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning