Consider the following reaction: 2CuCl2 (9) → 2CUCI Cl2 (3) (s) An initial mass of CuCl, (mol. wt. 134.4 g/mol) is reacted to produce Cl, gas. The final volume of gas collected after the reaction is 2.46 L. The temperature is 300K and the pressure 1 atm. How many grams of CuCl2 were reacted? O 14.2 g O 7.09 g 26.9 g O 6.72 g O 13.4 g
Consider the following reaction: 2CuCl2 (9) → 2CUCI Cl2 (3) (s) An initial mass of CuCl, (mol. wt. 134.4 g/mol) is reacted to produce Cl, gas. The final volume of gas collected after the reaction is 2.46 L. The temperature is 300K and the pressure 1 atm. How many grams of CuCl2 were reacted? O 14.2 g O 7.09 g 26.9 g O 6.72 g O 13.4 g
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 141AP: Consider the following unbalanced chemical equation: 6H12O6(s) + O2(g) -> CO,(g) + H2O(g) What...
Related questions
Question
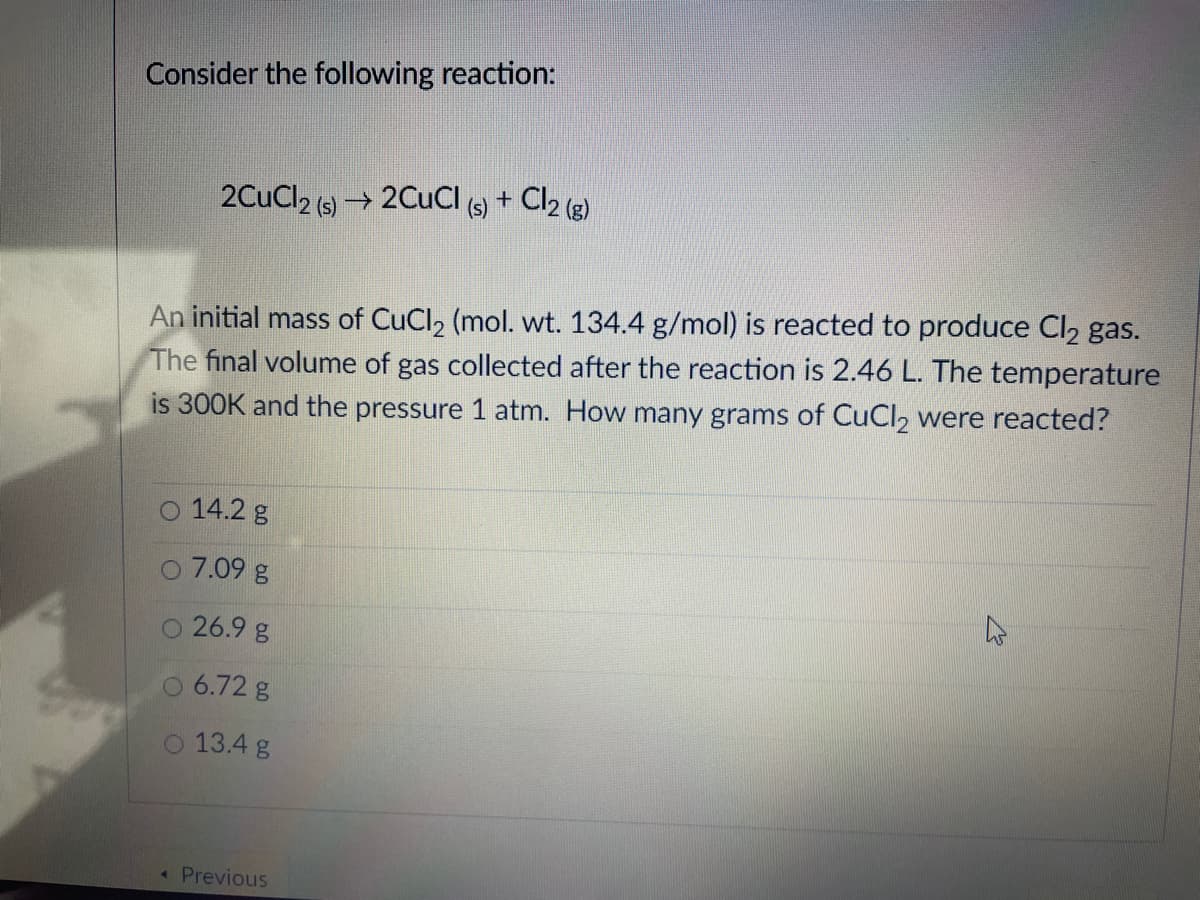
Transcribed Image Text:Consider the following reaction:
2CuCl2 (s) → 2CUCI +
Cl2 (e)
An initial mass of CuCl2 (mol. wt. 134.4 g/mol) is reacted to produce Cl2 gas.
The final volume of gas collected after the reaction is 2.46 L. The temperature
is 300K and the pressure 1 atm. How many grams of CuCl, were reacted?
O 14.2 g
O 7.09 g
26.9 g
O 6.72 g
O 13.4 g
« Previous
Expert Solution
Step 1
Reaction :-
2CuCl2(s) ------------> 2CuCl(s) + Cl2(g)
Molar mass of CuCl2 = 134.4g/mol
Pressure = 1atm
Temperature = 300K
Volume of gas = 2.46L
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning