Consider the following reaction at a certain temperature: 4 Fe(s) + 3 O2(g) → 2 Fe2O3(s) An equilibrium mixture contains 1.2 mol Fe, 0.0010 mol O2, and 2.0 mol Feɔ03 all in a 2.0-L container. Calculate the value of K for this reaction. K =
Consider the following reaction at a certain temperature: 4 Fe(s) + 3 O2(g) → 2 Fe2O3(s) An equilibrium mixture contains 1.2 mol Fe, 0.0010 mol O2, and 2.0 mol Feɔ03 all in a 2.0-L container. Calculate the value of K for this reaction. K =
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter12: Gaseous Chemical Equilibrium
Section: Chapter Questions
Problem 13QAP: Consider the following reaction at 250C: A(s)+2B(g)C(s)+2D(g) (a) Write an equilibrium constant...
Related questions
Question
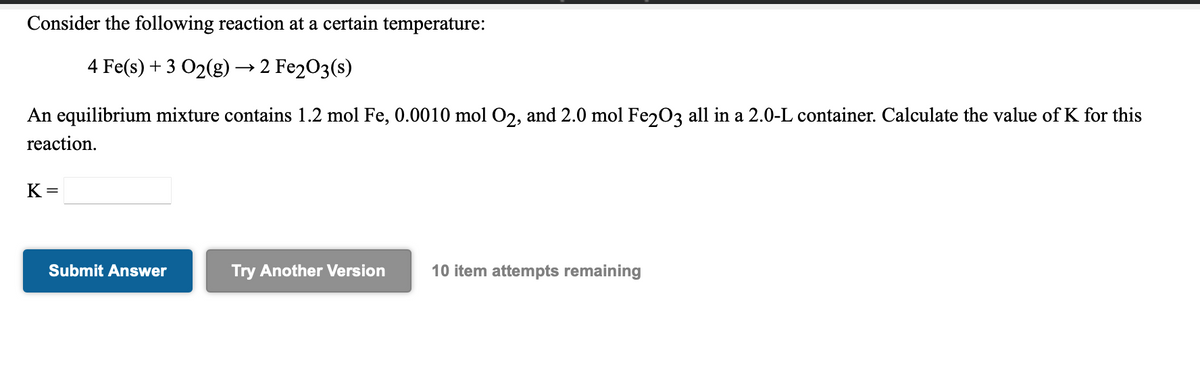
Transcribed Image Text:Consider the following reaction at a certain temperature:
4 Fe(s) + 3 O2(g) → 2 Fe2O3(s)
An equilibrium mixture contains 1.2 mol Fe, 0.0010 mol O2, and 2.0 mol Fe203 all in a 2.0-L container. Calculate the value of K for this
reaction.
K =
Submit Answer
Try Another Version
10 item attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax