Consider the following reaction where Kc = 10.5 at 350 K: 2 CH₂Cl₂ (9) CH4 (9) + CCI4 (9) A reaction mixture was found to contain 1.15x10-2 moles of CH₂Cl₂ (9), 5.27x10-2 moles of CH4 (9), and 3.67x10-2 moles of CCl4 (9), in a 1.00 liter container. Indicate True (I) or False (F) for each of the following: 1. In order to reach equilibrium CH₂Cl₂(g) must be consumed. 2. In order to reach equilibrium K must decrease 3. In order to reach equilibrium CH4 must be consumed. 4. Qc is less than Ke 5. The reaction is at equilibrium. No further reaction will occur.
Consider the following reaction where Kc = 10.5 at 350 K: 2 CH₂Cl₂ (9) CH4 (9) + CCI4 (9) A reaction mixture was found to contain 1.15x10-2 moles of CH₂Cl₂ (9), 5.27x10-2 moles of CH4 (9), and 3.67x10-2 moles of CCl4 (9), in a 1.00 liter container. Indicate True (I) or False (F) for each of the following: 1. In order to reach equilibrium CH₂Cl₂(g) must be consumed. 2. In order to reach equilibrium K must decrease 3. In order to reach equilibrium CH4 must be consumed. 4. Qc is less than Ke 5. The reaction is at equilibrium. No further reaction will occur.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter13: Fundamental Equilibrium Concepts
Section: Chapter Questions
Problem 12E: Show that the complete chemical equation, the total ionic equation, and the net ionic equation for...
Related questions
Question
4-5
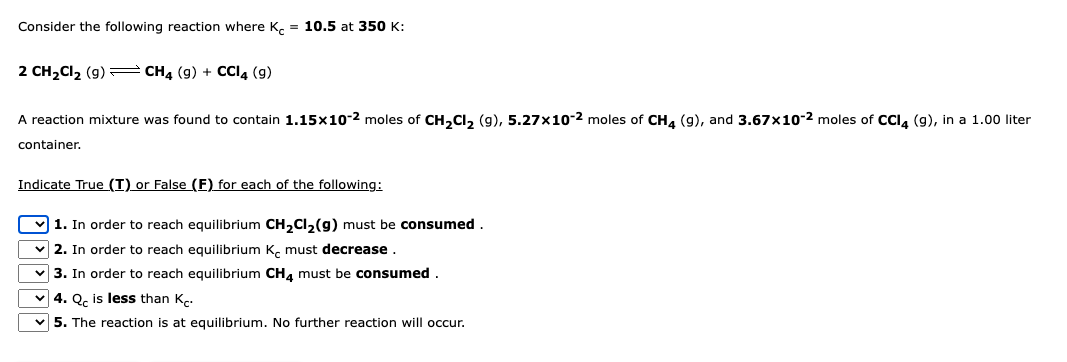
Transcribed Image Text:Consider the following reaction where Kc = 10.5 at 350 K:
2 CH₂Cl₂ (9) CH4 (9) + CCI4 (9)
A reaction mixture was found to contain 1.15x10-2 moles of CH₂Cl₂ (9), 5.27x10-2 moles of CH4 (9), and 3.67x10-2 moles of CCl4 (9), in a 1.00 liter
container.
Indicate True (I) or False (F) for each of the following:
1. In order to reach equilibrium CH₂Cl₂(g) must be consumed.
2. In order to reach equilibrium K must decrease
3. In order to reach equilibrium CH4 must be consumed.
4. Qc is less than K.
5. The reaction is at equilibrium. No further reaction will occur.
Expert Solution
Step 1
Given : equilibrium reaction
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning