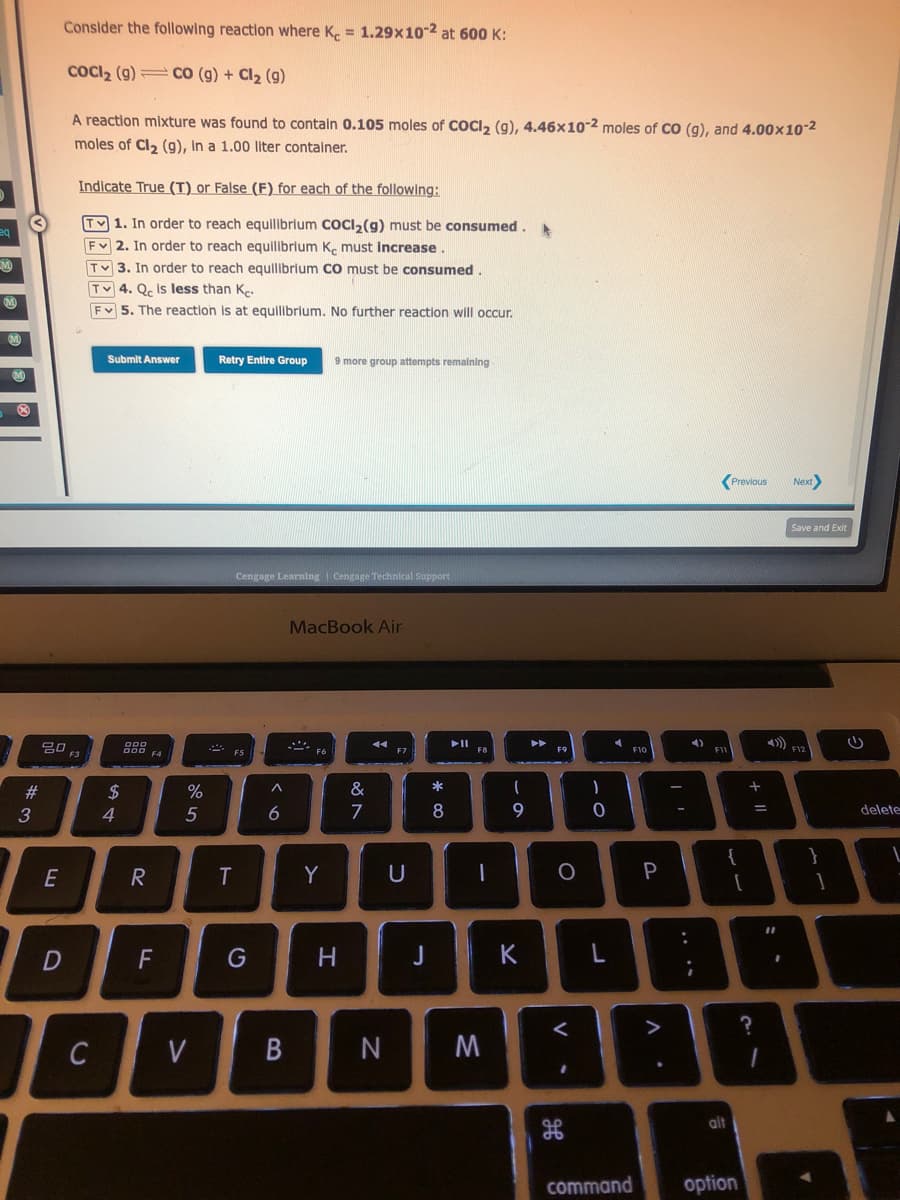
Consider the following reaction where Ke = 1.29x10-2 at 600 K: COCI₂ (9)CO (g) + Cl₂ (g) A reaction mixture was found to contain 0.105 moles of COCl₂ (g), 4.46x10-2 moles of CO (g), and 4.00×10-² moles of Cl₂ (g), In a 1.00 liter container. Indicate True (T) or False (F) for each of the following: T1. In order to reach equilibrium CoCl₂(g) must be consumed. F2. In order to reach equilibrium Ke must increase. TV3. In order to reach equilibrium CO must be consumed. TV 4. Qe is less than Ke. F 5. The reaction is at equilibrium. No further reaction will occur.
Consider the following reaction where Ke = 1.29x10-2 at 600 K: COCI₂ (9)CO (g) + Cl₂ (g) A reaction mixture was found to contain 0.105 moles of COCl₂ (g), 4.46x10-2 moles of CO (g), and 4.00×10-² moles of Cl₂ (g), In a 1.00 liter container. Indicate True (T) or False (F) for each of the following: T1. In order to reach equilibrium CoCl₂(g) must be consumed. F2. In order to reach equilibrium Ke must increase. TV3. In order to reach equilibrium CO must be consumed. TV 4. Qe is less than Ke. F 5. The reaction is at equilibrium. No further reaction will occur.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter18: Principles Of Chemical Reactivity: Entropy And Free Energy
Section18.4: Entropy Measurement And Values
Problem 3RC: Calculate rS for the following reaction at 25 C. 2 H2(g) + O2(g) 2 H2O() (a) 326.6 J/K mol-rxn...
Related questions
Question
Transcribed Image Text:eq
M
3
#3
Consider the following reaction where Ke = 1.29x10-2 at 600 K:
COCI₂ (g)CO (g) + Cl₂ (9)
A reaction mixture was found to contain 0.105 moles of COCl₂ (g), 4.46x10-2 moles of CO (g), and 4.00x10-2
moles of Cl₂ (g), in a 1.00 liter container.
20
E
D
Indicate True (T) or False (F) for each of the following:
TV 1. In order to reach equilibrium COCI₂(g) must be consumed. A
FV 2. In order to reach equilibrium Ke must increase
TV3. In order to reach equilibrium CO must be consumed.
Tv 4. Qc is less than Ke
Fv 5. The reaction is at equilibrium. No further reaction will occur.
F3
C
Submit Answer
$
4
000
000 F4
R
F
%
5
V
Retry Entire Group
F5
Cengage Learning Cengage Technical Support
J
6
T
G
^
B
9 more group attempts remaining
MacBook Air
F6
Y
H
&
7
44
F7
U
* 00
J
8
►II
F8
1
N M
- a
9
K
A
►►
F9
O
<
.
-0
)
4
F10
command
P
V
.
I'
4)
F11
alt
Previous
option
+
?
=
Next>
1
Save and Exit
F12
11
:
(
delete
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning