Consider the following table of binary ionic compounds, their names, and their formulas. Which row is incorrect? Atom 1 Atom 2 Compound formula Compound name Na Br NaBr sodium bromide II Mn MnO2 manganese(II) oxide II Ca CaS calcium sulfide IV Fe CI FeCl3 iron(III) chloride O IV O II
Consider the following table of binary ionic compounds, their names, and their formulas. Which row is incorrect? Atom 1 Atom 2 Compound formula Compound name Na Br NaBr sodium bromide II Mn MnO2 manganese(II) oxide II Ca CaS calcium sulfide IV Fe CI FeCl3 iron(III) chloride O IV O II
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter18: Representative Metals, Metalloids, And Nonmetals
Section: Chapter Questions
Problem 3E: Predict the formulas for the nine compounds that may form when each species in column 1 of Table...
Related questions
Question
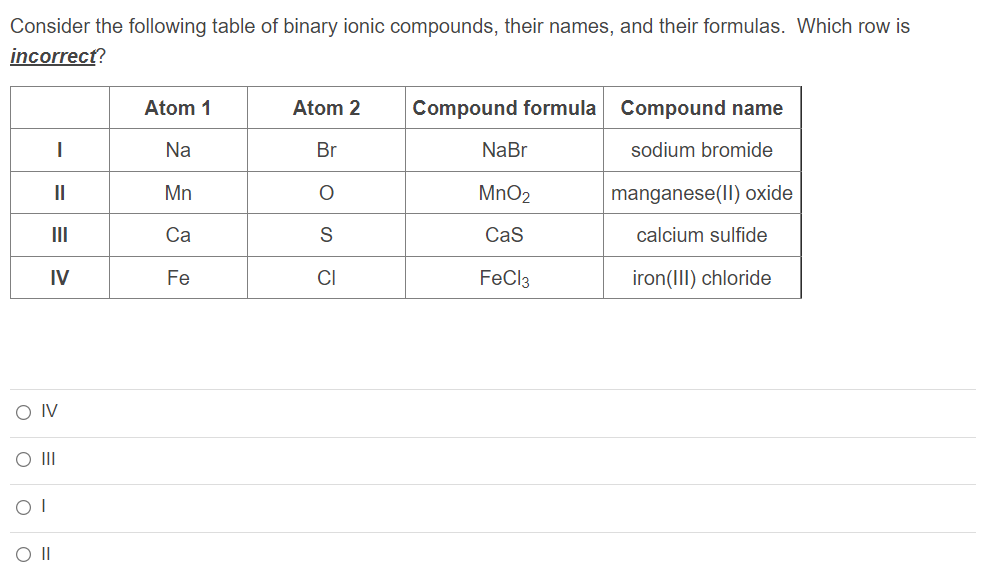
Consider the following table of binary ionic compounds, their names, and their formulas. Which row is incorrect?
| Atom 1 | Atom 2 | Compound formula | Compound name | |
| I | Na | Br | NaBr | sodium bromide |
| II | Mn | O | MnO2 | manganese(II) oxide |
| III | Ca | S | CaS | calcium sulfide |
| IV | Fe | Cl | FeCl3 | iron(III) chloride |
Group of answer choices
IV
III
I
II
Transcribed Image Text:Consider the following table of binary ionic compounds, their names, and their formulas. Which row is
incorrect?
Atom 1
Atom 2
Compound formula Compound name
Na
Br
NaBr
sodium bromide
II
Mn
MnO2
manganese(II) oxide
II
Ca
CaS
calcium sulfide
IV
Fe
CI
FeCl3
iron(III) chloride
O IV
O II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning
