Consider the reaction and its equilibrium constant at 25.0 ° C: I2(g) + Cl2 (g) =2ICI(g). K, = 81.9 A reaction mixture contains P(I2) = 0.281 atm, P(Cl2) = 0.281 atm, and P(ICI) = 0.281 atm. Is the reaction mixture at equilibrium? %3D %3D
Consider the reaction and its equilibrium constant at 25.0 ° C: I2(g) + Cl2 (g) =2ICI(g). K, = 81.9 A reaction mixture contains P(I2) = 0.281 atm, P(Cl2) = 0.281 atm, and P(ICI) = 0.281 atm. Is the reaction mixture at equilibrium? %3D %3D
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 51P
Related questions
Question
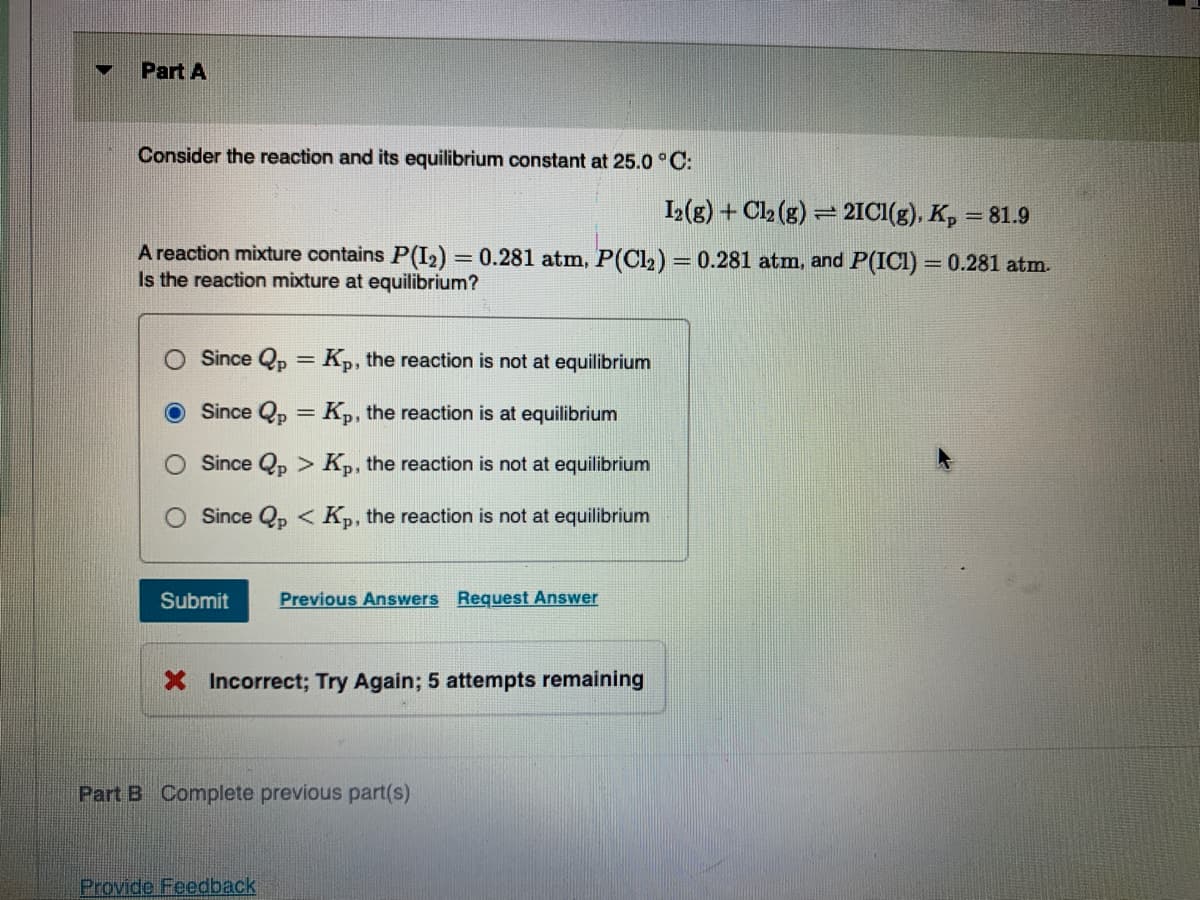
Transcribed Image Text:Part A
Consider the reaction and its equilibrium constant at 25.0 ° C:
I2(g) + Cl2 (g) = 2IC1(g). K, = 81.9
A reaction mixture contains P(I2) = 0.281 atm, P(Cl2) = 0.281 atm, and P(ICI) = 0.281 atm.
Is the reaction mixture at equilibrium?
%3D
Since Qp = K,, the reaction is not at equilibrium
%3D
Since Qp = K,, the reaction is at equilibrium
Since Qp > Kp, the reaction is not at equilibrium
O Since Qp < Kp, the reaction is not at equilibrium
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 5 attempts remaining
Part B Complete previous part(s)
Provide Feedback
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning