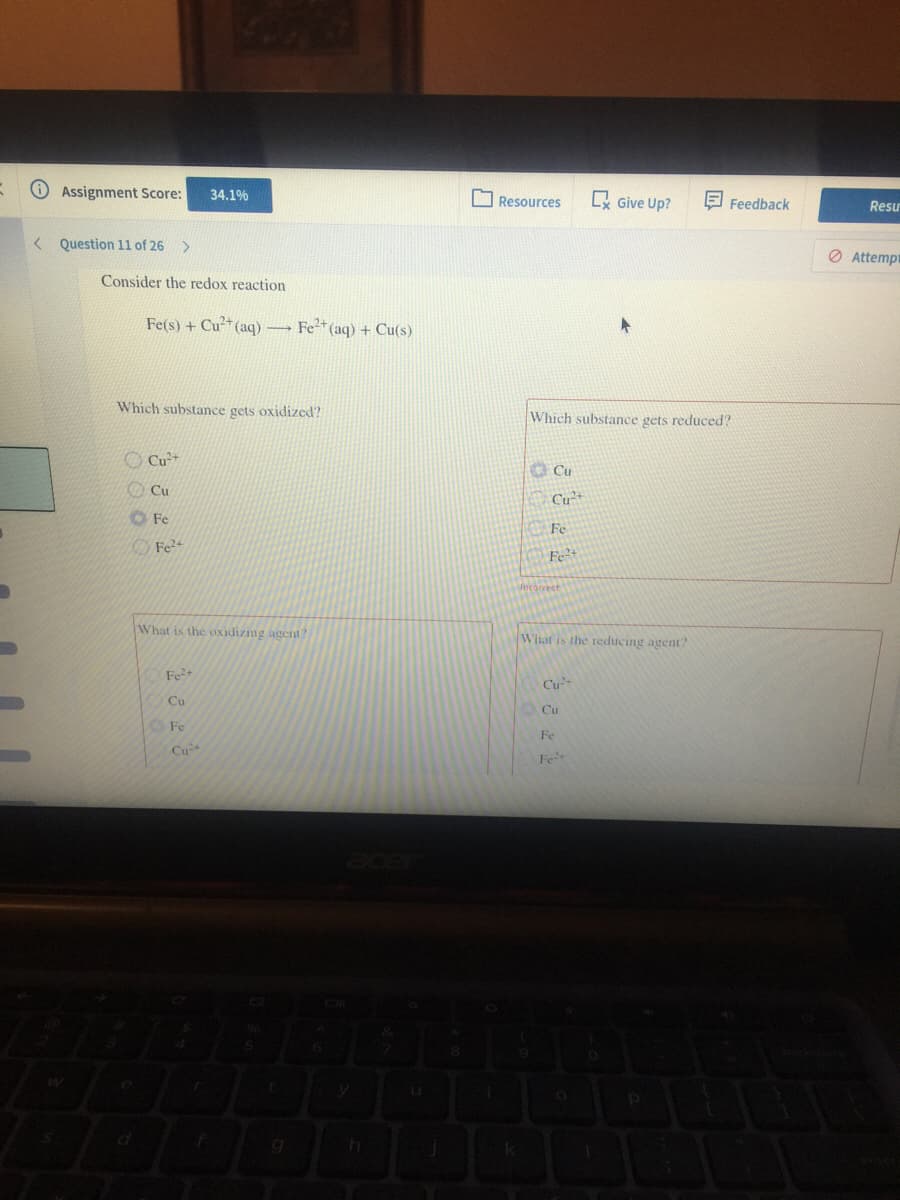
Consider the redox reaction Fe(s) + Cu* (aq) Fe* (aq) + Cu(s) - Which substance gets oxidized? Which substance gets reduced? Cu O Cu OCu Cu*+ OFe Fe Fe Fe²+ caect What is the oxidizing agent? What is the reducing agent
Q: Which of the following metals can be used to reduce iron in an aqueous solution of iron(III)…
A: Concept- According to the metal reactivity series those metals have good reducing nature is capable…
Q: Decide whether each chemical reaction in the table below is an oxidation-reduction ("redox")…
A: Oxidation ; The reaction in which substance lose the electron is called as oxidation reaction . (…
Q: Which one of the following represents the net ionic equation for the reaction of Ni(C,H,O,), with…
A:
Q: Consider the following reaction: Mg (aq) + Cu(s) → Cu2"(aq) + Mg(s). In this reaction, Mg2 (aq) is:…
A: We know that, Oxidation means loss of electron . Reduction means gain of an electron. Reducing…
Q: In the reaction shown below, the oxidizing agent is ____. 3 Fe(NO3)2 (aq) + 2 Al (s) → 3 Fe (s) + 2…
A: the oxidizing agent is an agent that oxidizes other substances and reduced otself.
Q: Is this a redox reaction? Pb2+(aq) + S2- → PbS (s) Is this a redox reaction? Li (s) + H2O (l) →…
A: The reactions which involve oxidation and subsequent reduction of different species is called a…
Q: What is the oxidation number of iron, Fe, in Fe(NH4)2SO4?
A:
Q: Identify the oxidized substance, the reduced substance, the oxidizing agent, and the reducing agent…
A: Oxidation = When the substance in the reaction loss electrons then this is called as oxidation . (…
Q: Which of the following metals can be used to reduce iron in an aqueous solution of iron(II)…
A: According to the metal reactivity series, the metals which have good reducing nature compare then Fe…
Q: Cu(s) + NO3-(aq) → Cu2+(aq) + NO2(g) Referring to the equation above, which is the reducing agent?
A: A reducing agent is a species in the chemical reaction that reduces others and gets oxidized itself.
Q: Classify the following reaction: Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s) a. oxidation-reduction…
A: An oxidation-reduction (redox) reaction is a type of chemical reaction that involves a transfer of…
Q: In the following chemical reaction, which element is the reducing agent? 2 IO₃⁻(aq) + 12 H⁺(aq) + 10…
A: The question is based on the concept of redox reactions. these are the reactions in which…
Q: In the following reaction, the oxidation number of O changes from to Fe,O,+ 3CO → 2Fe+ 3CO, a)…
A: The oxidation number of O doesn’t change in fallowing reaction Fe2O3 + 3CO ——> 2Fe + 3CO2…
Q: Decide whether each chemical reaction in the table below is an oxidation-reduction ("redox")…
A: Redox reaction: A reaction which is accompanied by change in oxidation state of elements. Oxidation:…
Q: What element is being oxidized in the following redox reaction? FeCl3 + CuCl -----> FeCl2…
A:
Q: Identify the oxidized substance and the reduced substance in the following redox reaction: Cu(s) + 2…
A: The reaction in which one substance get oxidized means the oxidation state of one substance…
Q: 4. In the following reaction, what substance loses electrons? 2 Fe (aq) + Sn2 (aq) → 2 Fe2"(aq) +…
A:
Q: In the reaction 2 HCl(aq) + Zn(s) ⟶ZnCl2(aq) + H2(g), what is the reducing agent and what is the…
A: Oxidising agent : The substance that is reduced in the reaction is the oxidizing agent because it…
Q: Mg(s) + HBr (aq)→ Zn(s) + Ni(NO,)a (aq) → Ca(s) + H,SO, (aq)→ Write an equation for the reaction if…
A: Balance Chemical equation means no of atoms or ions should be equaal in both side reactant and…
Q: Write the net ionic equation for the reaction of copper(II) sulfate with sodium sulfide. A) 2 Cu*…
A:
Q: In the following reaction, which element in what species is oxidized? Au(NO,), (aq) + 3 Li(s) →…
A: A redox reaction or oxidation/reduction reaction is the reaction in which one species undergo…
Q: Consider the reaction Al(s) + Zr**(aq) – Al*(aq) + Zr(s) Which of the following statements is FALSE?…
A:
Q: In the following reaction which occurs from left to right: 3 Cu2*(aq) + 2 Fe(s) → 2 Fe3*(aq) + 3…
A: Introduction: Oxidation: Oxidation is the loss of electrons or hydrogen atoms or gain of oxygen…
Q: In the process of pickling, rust is removed from newly pro-duced steel by washing the steel in…
A: “Since there are multiple sub-parts in this question and it is not mentioned that which one has to…
Q: Consider the following reaction: Pb2+ (aq) + Sn (s) → Pb (s) + Sn²+ (aq) In the reaction as written,…
A: Given :- Pb2+(aq) + Sn(s) → Pb(s) + Sn2+(aq) To identify :- Reducing agent
Q: In the following reaction, Rb is 2 Rb(s) + Cl2(g) - 2 RBCI(s) A) the reducing agent. B) reduced. O…
A:
Q: Consider the redox reaction Fe(s) + Cu²* (aq) → Fe²* (aq) + Cu(s) Which substance gets oxidized?…
A:
Q: oxidizing agent and the reducing agent 3 + Ag* + H20 + NaBr nese metal reacts with HCl to give…
A: In the balanced chemical reaction number of atoms of each element in product side is equal to number…
Q: A sample of an iron ore is dissolved in acid, and the iron isconverted to Fe2+. The sample is then…
A:
Q: Consider the redox reaction Fe(s) + Cu2+(aq) -----> Fe2+(aq) + Cu(s) Which substance gets…
A: Before solving this question we must know the following terminology:(a) Oxidising agent:→Oxidizing…
Q: A displacement reaction is an oxidation-reduction reaction in which a free element reacts with a…
A:
Q: Write the net ionic equation for the reaction of copper(Il) sulfate with sodium sulfide. A) 2 Cu*…
A:
Q: I. What is oxidation? What is reduction? II. What are the oxidation states of Cu and Zn (both…
A: Given is redox reaction.
Q: Decide whether each chemical reaction in the table below is an oxidation-reduction ("redox")…
A:
Q: In the reaction of dichromate ion and iodide ion: Cr2O72-(aq) + 14 H+(aq) + 6 I-(aq) ---> 2…
A: The balanced reaction taking place is given as, => Cr2O72-(aq) + 14 H+(aq) + 6 I-(aq) ---> 2…
Q: Calcium metal and copper(II) nitrate solution are mixed. Over time the blue solution slowly becomes…
A: 1. Calcium is more reactive than copper so it will replace Cu to form calcium nitrate. Calcium is…
Q: For the reaction: 3 Mg) + Ne)- Mg N0) YWhich species is reduced? (A) Mg (B) N (C) MgN 2 Which…
A: The species whose oxidation number is decreased is reduced itself and oxidized others. The species…
Q: e. KF(aq) +1, (s) - Gre the products and balance the following double displacement reactions. Assume…
A: Double displacement reaction: In double displacement reaction the cation and anion of the two…
Q: Which of the following equations represents an oxidation-reduction reaction? Ca(OH), (s) + H,So,…
A: In a redox reaction, one species get oxidised and other get reduced via the transfer of electrons…
Q: In the following redox reaction, what are the reducing agent, and the oxidizing agent, respectively?…
A: The redox reaction given is Cl2 (aq) + 2 Fe2+ (aq) → 2 Cl‾ (aq) + 2 Fe3+ (aq) Reducing agents are…
Q: What is the oxidizing and reducing agent in the balanced equation? Mg(s) + Cr(C2H3O2)3(aq) --->…
A: The given reaction is : Mg(s) + Cr(C2H3O2)3(aq) → Mg(C2H3O2)3 (aq) + Cr(s) Oxidising agent is one…
Q: FeO + Zn(s) --------->. ZnO + Fe(s) What is the Oxidizing Agent?
A: As per the classical concept on redox reactions, a redox reaction is the one that involves…
Q: Hydrogen peroxide is an effective oxidizing agent, accepting two electrons. It will oxidizediron(II)…
A: Redox reaction are those reactions in which oxidation (loss of electrons) and reduction (gain of…
Q: 1. Consider the following Redox reaction, 2+ 2Ag" (aq) + Cu(s) → 2Ag(s) + Cu“ (aq) The oxidizing…
A:
Q: Identify the oxidized substance, the reduced substance, the oxidizing agent, and the reducing agent…
A: Given :- Cu(s) + 2AgNO3(aq) → 2Ag(s) + Cu(NO3)2(aq) To determine :- Species oxidized Species…
Q: Decide whether each chemical reaction in the table below is an oxidation-reduction ("redox")…
A:
Q: Identify the attached reaction as either an acid-base (neutralization), precipitation, or…
A:
Q: 9. When iron filings are added to hydrochloric acid solution, H2 gas can be seen bubbling out of…
A: When dilute Hydrochloric acid is added to iron filings, iron(II) chloride & hydrogen gas is…
Q: In the following chemical reaction, which element is the reducing agent? 3 Sn(NO₃)₂(aq) + 2 Fe(s) →…
A: The element which helps in reduction of the other element is known as reducing agent And since the…
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

- 7. Consider a1Msolution ofNa3AsO4. Write the charge and mass balance equations for this system. (please type answer not write by hend)Which of the following are equivalent to 2,500 ppm Cu2+? (There may be more than one answer) MW: Cu (63.55) a) 2.5 ppb Cu2+ b) 2,500,000 ppb Cu c) 2.5 ppt Cu2+ d) 39.34 mM e) 0.03934 M f) 0.07868 N (in precipitation reaction) g) 0.07868 N (in redox into Cu+)Ochem help with prelab The experiment is attached and involves the reduction of a nitro group to an amine The Chemicals are: 200 mg of m-nitroacetophenone 400 mg of granular tin 4 mL of 6M hydrochloric acid 30% sodium hydroxide Need help with the prelab which requires... A. Write the balanced equations for both half-reactions of the redox and then the overall balanced redox equation B. Determine the limiting reagent and the theoretical yield of amine
- In basic solution, molecular chlorine, Cl2, reacts with hydroxide ions, OH^-, to yield chloride ions, Cl^-. and chlorate ions, ClO4^-. Hint: This is a disproportionation reaction in which the element chlorine is both oxidized and reduced. Write a balanced equation for this reaction. Answer: 3Cl2 + 6OH^- ---> 5Cl^- + ClO3^- + 3H2O Show work?The iodine produced when excess of KI was added to the solution containing 0.1259 g K2Cr2O7 required a 41.26 mL titration with Na2S2O3 required a 41.26 mL titration with Na2S2O3. P.S. Be able to answer numbers 1 and 2 ONLY. 1. Determine if it is basic or acidic and write the balance redox reaction for the reaction of dichromate ion with iodide ion in acidic medium. Unbalance equation: Cr2O72- (aq) + I- (aq) → Cr3+ + I2 (aq) 2. Determine if it is basic or acidic and write the balance redox reaction of iodine with sodium thiosulfate in acidic medium. Unbalance equation: S2O3 2- (aq) + I2 (aq) → S4O6 2- (aq) + I- (aq) 3. (i) What are the oxidizing agents in #1 and #2? (ii) What is the stoichiometric ratio between dichromate and thiosulfate? 4. Calculate the molar concentration of the thiosulfate solution.Reaction: CrO42-(aq)+2H+(aq) <--> Cr2O72-(aq)+H2O(l) YELLOW ORANGE a) Under what conditions will Cr2O72-(aq) predominate? explain / defend your answer choice. b) is this a redox reaction? explain/ defend your answer.
- A 1.963-g sample of an alloy is dissolved in HNO3 and diluted to volume in a 100-mL volumetric flask. Titrating a 25.00-mL portion with 0.1078 M KSCN requires 27.19 mL to reach the end point. Calculate the %w/w Ag in the alloy. (Mwt of Ag = 107.87 g/mol)Complete the neutralization reaction of phosphoric acid reacting with potassium hydroxide to form water and potassium phosphate. Input the coefficients of the reactancts and products, including 1, if applicable. Also, input the formula for the potassium phosphate in the last field. ?H3PO4 + ?KOH ---> ?H2O + ? & ?15. Calculate the reserve capacity at 80°F of an automobile battery that was discharged at 87°F for 102min.?A. 94.62 minB. 94.86 minC. 95.40 minD. 95.15 min Obs: I inform you that the answer to letter " C " is wrong, according to the test I did. I appreciate your help which of this question is the correct answer. thanks
- How many electrons are being transfer in the following unbalanced reaction Fe + O2 --> Fe2O3?Given the following unbalanced response:NO2-+MnO4-+H+ NO3-+Mn2++H2OWhat is the number of electrolyte molecules passing through the reaction? A. 1 B. 2 C. 5 D. 10IDENTIFY THE OXIDATION NUMBER OF EACH ELEMENT IN THE GIVEN CHEMICAL FORMULA. All answers to be entered via short answer are in the numerical format Include the sign (either positive or negative) E.g.: KI- What is the oxidation number of potassium?- Answer: +1- What is the oxidation number of iodine?- Answer: -1 Givenproblems: -What is the oxidation number of SODIUM in sodium chloride (NaCl)? -What is the oxidation number of CHLORINE in sodium chloride? -What is the oxidation number of MAGNESIUM in magnesium hydroxide Mg(OH)₂? -What is the oxidation number of OXYGEN in magnesium hydroxide Mg(OH)₂? -What is the oxidation number of HYDROGEN in magnesium hydroxide Mg(OH)₂? -What is the oxidation number of POTASSIUM in potassium dichromate K₂Cr₂O₇? -What is the oxidation number of CHROMIUM in potassium dichromate K₂Cr₂O₇? -What is the oxidation number of OXYGEN in potassium dichromate K₂Cr₂O₇? Given Problem: Identify the coefficient of each compound in the reaction. - All answers to…



