Considering the above information, which of these chemical reactions would AGbe approximately equal to AH? Choose one or more: Br + Br Br OCH3 + HOCH3+ Br LOTS + CN CN + TsO HCI
Considering the above information, which of these chemical reactions would AGbe approximately equal to AH? Choose one or more: Br + Br Br OCH3 + HOCH3+ Br LOTS + CN CN + TsO HCI
Chapter15: Complex Acid/base Systems
Section: Chapter Questions
Problem 15.6QAP
Related questions
Question
Changing the temperature of a reaction system can sometimes influence which type of reaction occurs, leading to different products at higher reaction temperatures than at lower. One of the most important variables in determining whether a reaction pathway will be influenced by temperature is the entropy change of the system. In many organic reactions, the entropy change of the reaction is close to zero, meaning that the TΔS term becomes negligible in the Gibbs free energy expression (ΔG = ΔH – TΔS). In these cases, ΔG is approximately equal to ΔH.
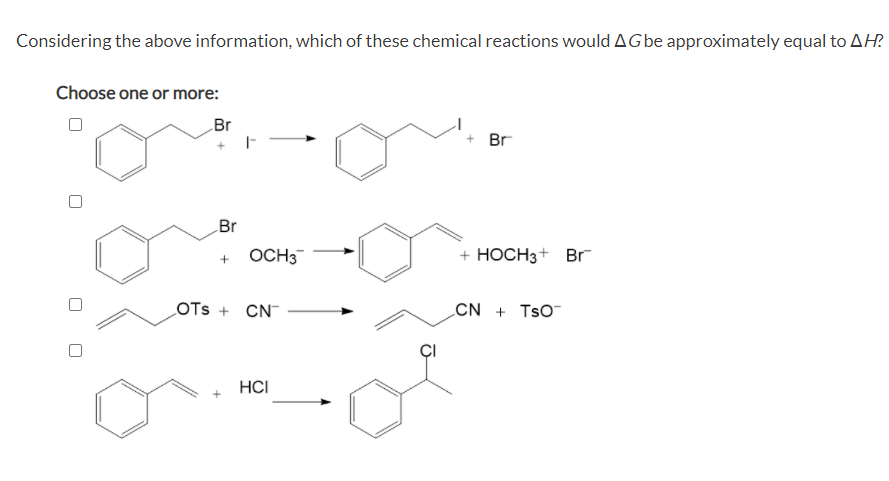
Transcribed Image Text:Considering the above information, which of these chemical reactions would AGbe approximately equal to AH?
Choose one or more:
Br
+ Br
Br
OCH3
+ HOCH3+ Br
LOTS + CN
CN + TsO
HCI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you



Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax



Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning