1. The magnitude of any enthalpy change depends on the conditions of temperature, pressure and state of the reactants and products. The more disordered the system, the smaller the entropy. 2. ASsys > 0 is an equation that represents the Second Law of Thermodynamic The equation Sgas = 0 represents the entropy of the substance. 3. Entropy changes cannot be measured with a calorimeter like enthalpy changes. If the result upon calculation of entropy changes in the universe is a positive value, the process is reactant-favored.
1. The magnitude of any enthalpy change depends on the conditions of temperature, pressure and state of the reactants and products. The more disordered the system, the smaller the entropy. 2. ASsys > 0 is an equation that represents the Second Law of Thermodynamic The equation Sgas = 0 represents the entropy of the substance. 3. Entropy changes cannot be measured with a calorimeter like enthalpy changes. If the result upon calculation of entropy changes in the universe is a positive value, the process is reactant-favored.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter16: Thermodynamics: Directionality Of Chemical Reactions
Section16.3: Measuring Dispersal Of Energy: Entropy
Problem 16.3CE
Related questions
Question
100%
Write the CAPITAL Letter of the correct answer and explain why
A if both statements are true
B if the 1st statement is false and the 2nd is true
C if the 1st statement is true and the 2nd is false
D if both statements are false

Transcribed Image Text:5. Changes that seem to happen by themselves in one direction are spontaneous reactions.
The entropy of the universe decreases in a spontaneous process.
6. A portion that is singled out in a study where energy can be transferred back and forth is called the
surroundings.
A thermochemical system where neither energy can be exchanged with its surroundings is called a
closed system.
7. Compounds formed from their elements in exothermic reaction have negative AH;
lonic compounds usually have more negative enthalpies of formation.
8. Thermodynamics can tell us the direction and extent of a reaction but tells nothing about the speed
of a reaction.
Majority of spontaneous reactions are exothermic especially in irreversible reactions.
9. Enthalpy provides a quantitative measure of how much energy is spread out when something
happens.
Entropy is associated with the second law of thermodynamics.
10. If the change in free energy, AG is negative, the reaction is spontaneous in the forward direction.
It is more convenient to use change in enthalpy, AH, as a criterion of spontaneity than change in
entropy, AS.
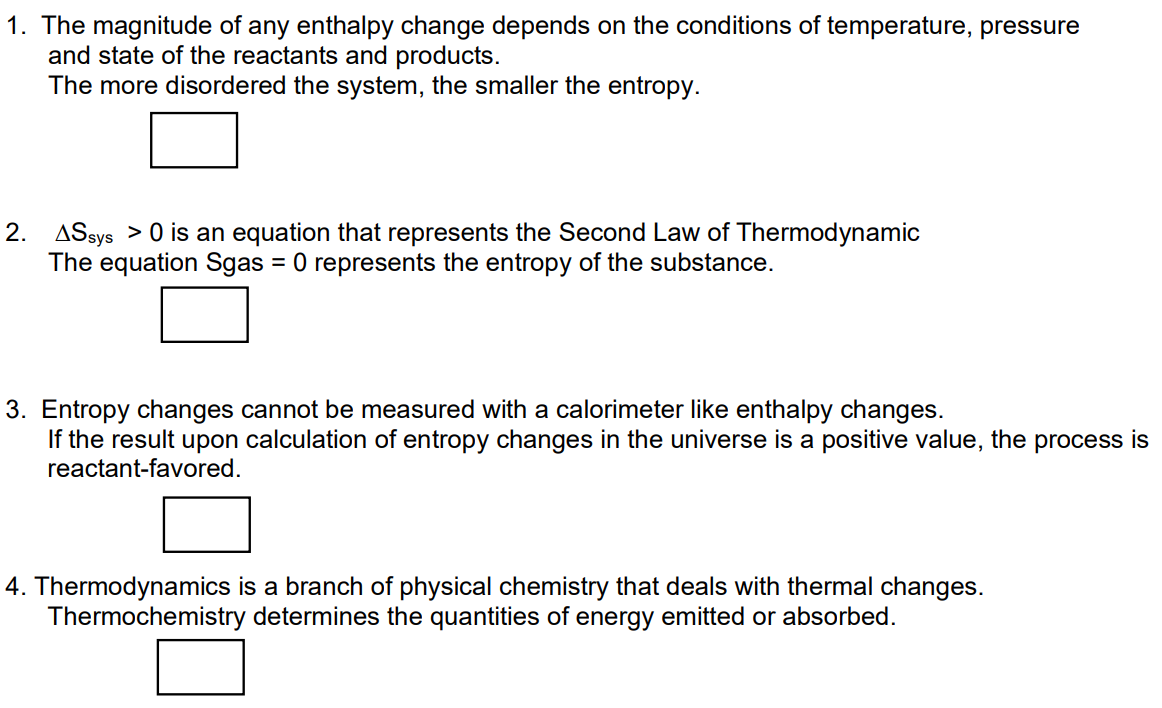
Transcribed Image Text:1. The magnitude of any enthalpy change depends on the conditions of temperature, pressure
and state of the reactants and products.
The more disordered the system, the smaller the entropy.
2. ASsys > 0 is an equation that represents the Second Law of Thermodynamic
The equation Sgas = 0 represents the entropy of the substance.
3. Entropy changes cannot be measured with a calorimeter like enthalpy changes.
If the result upon calculation of entropy changes in the universe is a positive value, the process is
reactant-favored.
4. Thermodynamics is a branch of physical chemistry that deals with thermal changes.
Thermochemistry determines the quantities of energy emitted or absorbed.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax