Constants Ocean thermal energy conversion is a process that uses the temperature difference between the warm surface water of tropical oceans and the cold deepocean water to run a heat engine. (Figure 1) shows a typical decrease of temperature with depth below the surface in tropical oceans. In the heat engine, the warmer surface water Part A vaporizes a low-boiling-point fluid, such as ammonia. The of amr Compare the entropy change of the warmer water to that of the colder water during one cycle of the heat engine, assuming an ideal Carnot cycle. heat of vaporization of ammonia is 260 cal/g at 27° C, the surface-water temperature. The vapor is used to turn turbine and is then condensed back into a liquid by means of cold water brought from deep below the surface through a large intake pipe. A power plant producing 10 MW of useful power would require a cold seawater flow rate of about 30,000 kg/s. O The entropy does not change during one cycle in either case. The entropy of the warmer water decreases by more than the entropy of the colder water increases, because some of the heat removed from the warmer water goes to the work done by the engine. The entropy of the warmer water decreases by the same amount that the entropy of the colder water increases. Figure 1 of 1 The entropy of both increases, but the entropy of the colder water increases by more because its initial temperature is lower. 5 10 15 20 25 30 °C 100 Submit Request Answer 200 300 400 Provide Feedback Next > 500 600 700 800 900 1000 Depth (m)
Constants Ocean thermal energy conversion is a process that uses the temperature difference between the warm surface water of tropical oceans and the cold deepocean water to run a heat engine. (Figure 1) shows a typical decrease of temperature with depth below the surface in tropical oceans. In the heat engine, the warmer surface water Part A vaporizes a low-boiling-point fluid, such as ammonia. The of amr Compare the entropy change of the warmer water to that of the colder water during one cycle of the heat engine, assuming an ideal Carnot cycle. heat of vaporization of ammonia is 260 cal/g at 27° C, the surface-water temperature. The vapor is used to turn turbine and is then condensed back into a liquid by means of cold water brought from deep below the surface through a large intake pipe. A power plant producing 10 MW of useful power would require a cold seawater flow rate of about 30,000 kg/s. O The entropy does not change during one cycle in either case. The entropy of the warmer water decreases by more than the entropy of the colder water increases, because some of the heat removed from the warmer water goes to the work done by the engine. The entropy of the warmer water decreases by the same amount that the entropy of the colder water increases. Figure 1 of 1 The entropy of both increases, but the entropy of the colder water increases by more because its initial temperature is lower. 5 10 15 20 25 30 °C 100 Submit Request Answer 200 300 400 Provide Feedback Next > 500 600 700 800 900 1000 Depth (m)
Chapter1: Temperature And Heat
Section: Chapter Questions
Problem 111AP: In some countries, liquid nitrogen is used on daily trucks instead of mechanical refrigerators. A...
Related questions
Question
100%
Transcribed Image Text:Constants
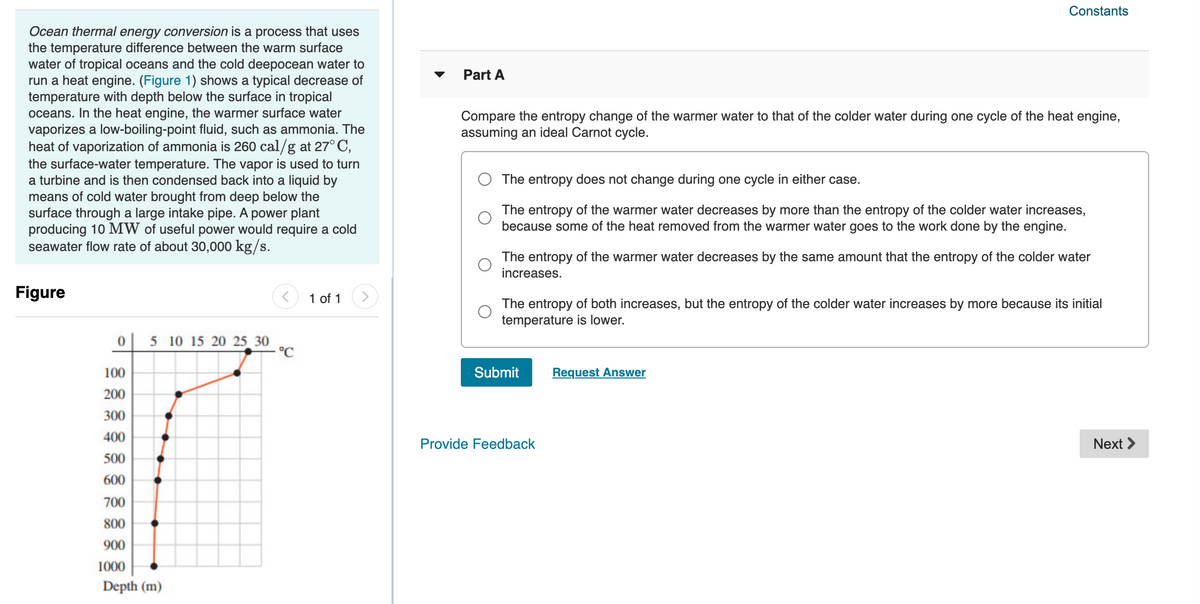
Ocean thermal energy conversion is a process that uses
the temperature difference between the warm surface
water of tropical oceans and the cold deepocean water to
run a heat engine. (Figure 1) shows a typical decrease of
temperature with depth below the surface in tropical
oceans. In the heat engine, the warmer surface water
vaporizes a low-boiling-point fluid, such as ammonia. The
heat of vaporization of ammonia is 260 cal/g at 27°C,
the surface-water temperature. The vapor is used to turn
a turbine and is then condensed back into a liquid by
means of cold water brought from deep below the
surface through a large intake pipe. A power plant
producing 10 MW of useful power would require a cold
seawater flow rate of about 30,000 kg/s.
Part A
Compare the entropy change of the warmer water to that of the colder water during one cycle of the heat engine,
assuming an ideal Carnot cycle.
The entropy does not change during one cycle in either case.
The entropy of the warmer water decreases by more than the entropy of the colder water increases,
because some of the heat removed from the warmer water goes to the work done by the engine.
The entropy of the warmer water decreases by the same amount that the entropy of the colder water
increases.
Figure
1 of 1
The entropy of both increases, but the entropy of the colder water increases by more because its initial
temperature is lower.
5 10 15 20 25 30
°C
100
Submit
Request Answer
200
300
400
Provide Feedback
Next >
500
600
700
800
900
1000
Depth (m)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning