Constants Periodic Table Assume all temperatures to be exact, and neglect significant figures for small changes in dimension. On a warm day (92 °F), an air-filled balloon occupies a volume of 0.300 m³ and has a pressure of 24.0 lb/in?. Part A If the balloon is cooled to 32 °F in a refrigerator while its pressure is reduced to 14.7 lb/in?, what is the volume of the air in the container? (Assume that the air behaves as an ideal gas.) nνα ΑΣφ V = 0.170 m3 Submit Previous Answers Request Answer X Incorrect; Try Again; 6 attempts remaining
Constants Periodic Table Assume all temperatures to be exact, and neglect significant figures for small changes in dimension. On a warm day (92 °F), an air-filled balloon occupies a volume of 0.300 m³ and has a pressure of 24.0 lb/in?. Part A If the balloon is cooled to 32 °F in a refrigerator while its pressure is reduced to 14.7 lb/in?, what is the volume of the air in the container? (Assume that the air behaves as an ideal gas.) nνα ΑΣφ V = 0.170 m3 Submit Previous Answers Request Answer X Incorrect; Try Again; 6 attempts remaining
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter13: Temperature, Kinetic Theory, And The Gas Laws
Section: Chapter Questions
Problem 59PE: Air in human lungs has a temperature of 37.0C and a saturation vapor density of 44.0g/m3. (a) If...
Related questions
Question
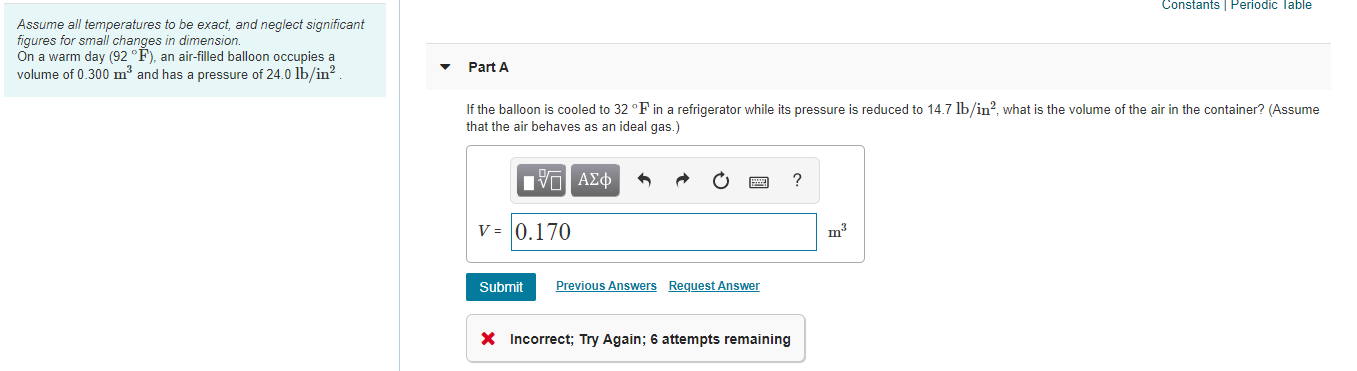
Transcribed Image Text:Constants Periodic Table
Assume all temperatures to be exact, and neglect significant
figures for small changes in dimension.
On a warm day (92 °F), an air-filled balloon occupies a
volume of 0.300 m³ and has a pressure of 24.0 lb/in?.
Part A
If the balloon is cooled to 32 °F in a refrigerator while its pressure is reduced to 14.7 lb/in?, what is the volume of the air in the container? (Assume
that the air behaves as an ideal gas.)
nνα ΑΣφ
V = 0.170
m3
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 6 attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill