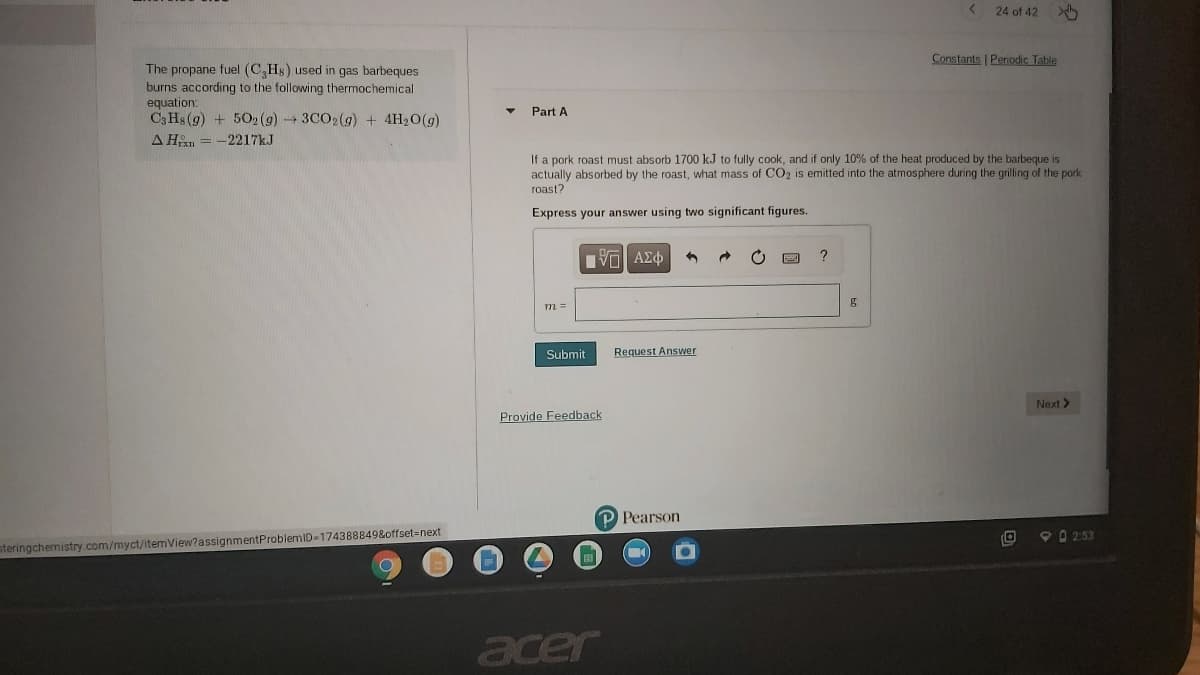
Constants Periodic Table The propane fuel (C,Hs) used in gas barbeques burns according to the following thermochemical equation: C3 Hs (g) + 502 (g)3CO2(g) + 4H20(g) A Hin = -2217kJ Part A If a pork roast must absorb 1700 k.J to fully cook, and if only 10% of the heat produced by the barbeque is actually absorbed by the roast, what mass of CO2 is emitted into the atmosphere during the grilling of the pork roast? Express your answer using two significant figures. ? m =
Constants Periodic Table The propane fuel (C,Hs) used in gas barbeques burns according to the following thermochemical equation: C3 Hs (g) + 502 (g)3CO2(g) + 4H20(g) A Hin = -2217kJ Part A If a pork roast must absorb 1700 k.J to fully cook, and if only 10% of the heat produced by the barbeque is actually absorbed by the roast, what mass of CO2 is emitted into the atmosphere during the grilling of the pork roast? Express your answer using two significant figures. ? m =
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 14P
Related questions
Question
Transcribed Image Text:24 of 42
Constants | Perodic Table
The propane fuel (C,Hs) used in gas barbeques
burns according to the following thermochemical
equation:
C3 Hs (g) + 502 (g)3CO2(g) + 4H20(g)
Part A
A Hn = -2217KJ
If a pork roast must absorb 1700 kJ to fully cook, and if only 10% of the heat produced by the barbeque is
actually absorbed by the roast, what mass of CO2 is emitted into the atmosphere during the grilling of the pork
roast?
Express your answer using two significant figures.
?
Πνα ΑΣφ
Submit
Request Answer
Next>
Provide Feedback
P Pearson
P 0 2:53
steringchemistry.com/myct/itemView?assignmentProblemID-174388849&offset-next
acer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning