Copper was determined in a river water sample by atomic absorption spectrometry and the method of standard additions. For the addition, 300.0 µL of a 1000.0-µg/mL Cu standard was added to 200.0 mL of solution. The following data were obtained: Absorbance of reagent blank = 0.013 Absorbance of sample = 0.457 Absorbance of sample plus addition - blank a Calculate the copper concentration in the sample. ✓ µg/mL Concentration = Correct 1.2 Error M = 1.014 Correct 4 b Later studies showed that the reagent blank used to obtain the above data was inadequate and that the actual blank absorbance was 0.100. Find the copper concentration with the appropriate blank, and determine the error caused by using an improper blank. Concentration = ug/mL %
Copper was determined in a river water sample by atomic absorption spectrometry and the method of standard additions. For the addition, 300.0 µL of a 1000.0-µg/mL Cu standard was added to 200.0 mL of solution. The following data were obtained: Absorbance of reagent blank = 0.013 Absorbance of sample = 0.457 Absorbance of sample plus addition - blank a Calculate the copper concentration in the sample. ✓ µg/mL Concentration = Correct 1.2 Error M = 1.014 Correct 4 b Later studies showed that the reagent blank used to obtain the above data was inadequate and that the actual blank absorbance was 0.100. Find the copper concentration with the appropriate blank, and determine the error caused by using an improper blank. Concentration = ug/mL %
Chapter12: Spectrochemical Methods
Section: Chapter Questions
Problem 12P
Related questions
Question
Chemistry
help please
keep getting part b wrong
keep getting part b wrong
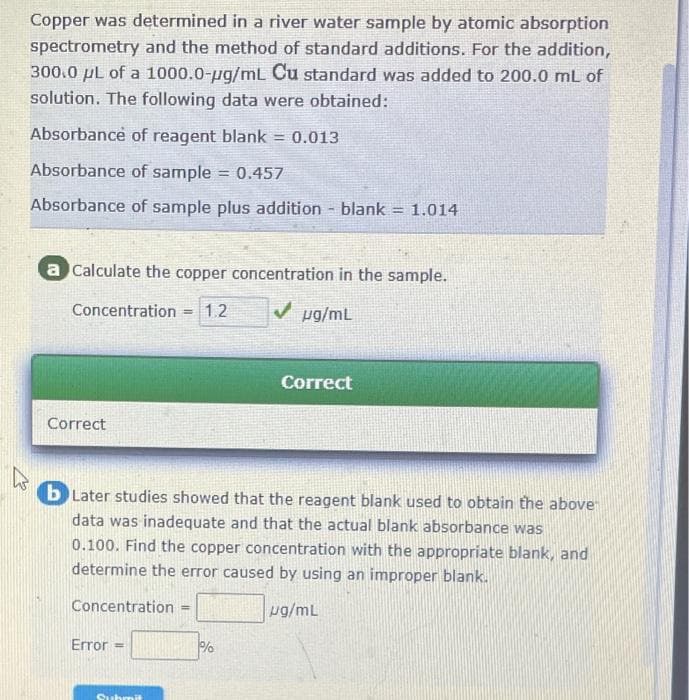
Transcribed Image Text:Copper was determined in a river water sample by atomic absorption
spectrometry and the method of standard additions. For the addition,
300.0 μL of a 1000.0-µg/mL Cu standard was added to 200.0 mL of
solution. The following data were obtained:
Absorbance of reagent blank = 0.013
Absorbance of sample = 0.457
Absorbance of sample plus addition - blank = 1.014
a Calculate the copper concentration in the sample.
Concentration =1.2
✓ µg/mL
Correct
bLater studies showed that the reagent blank used to obtain the above-
data was inadequate and that the actual blank absorbance was
0.100. Find the copper concentration with the appropriate blank, and
determine the error caused by using an improper blank.
Concentration
ug/mL
Error:
=
Submit
=
Correct
%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 8 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning