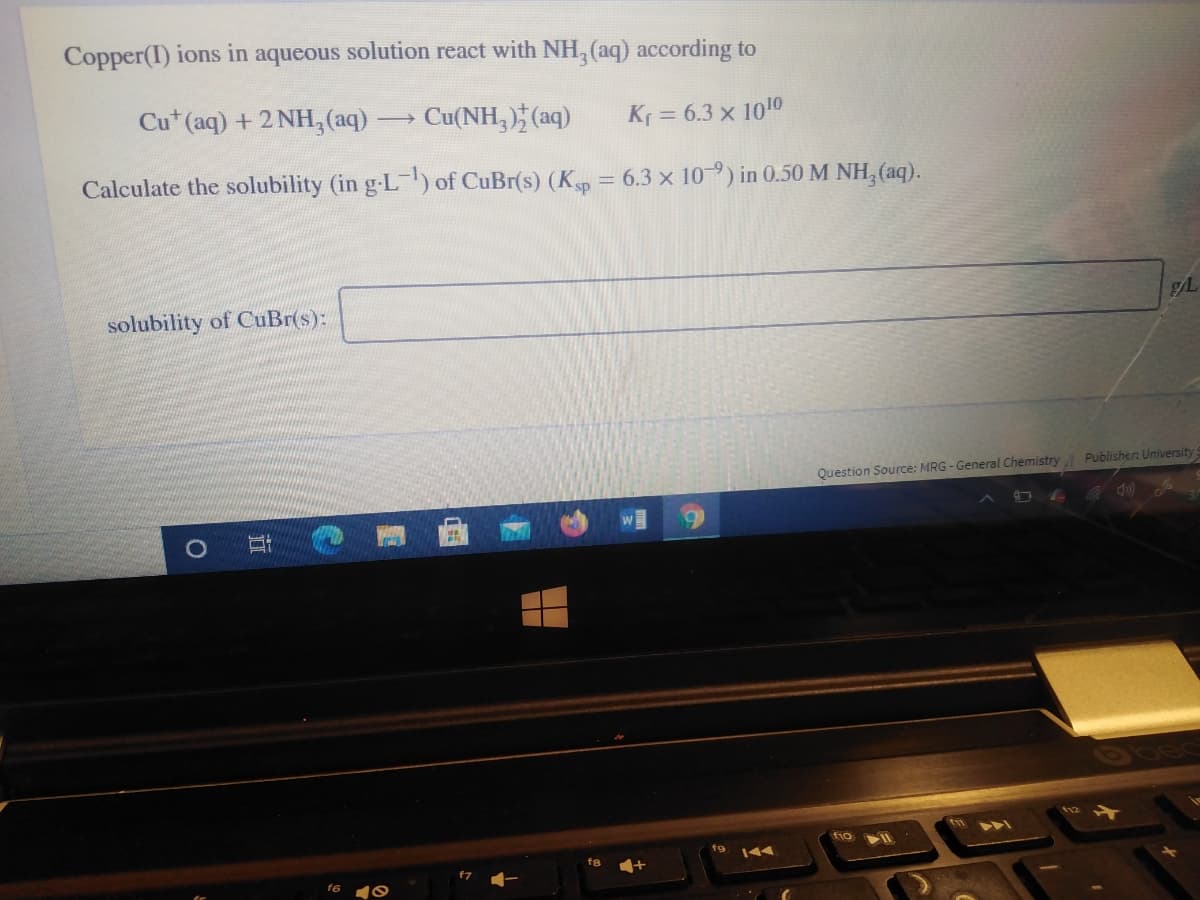
Copper(I) ions in aqueous solution react with NH, (aq) according to Cu* (aq) + 2 NH,(aq) Cu(NH,) (aq) Kr = 6.3 x 1010 Calculate the solubility (in g-L) of CuBr(s) (Kp = 6.3 x 10-9) in 0.50 M NH, (aq). %3D solubility of CuBr(s): Question Source: MRG - General Chemistry Publisher: University
Q: The Ksp of Cu3(PO4), is 1.00 × 10¬37. Estimate the solubility of this salt in units of g. L-1. You…
A: Given: The reaction for the dissociation of Cu3(PO4)2.(s).…
Q: Construct the expression for Ksp for solid A9CN in aqueous solution. AgCN(s) = Ag*(aq) + CN-(aq) 1…
A:
Q: Given that the solubility of BaC2O4 is s = 0.29 g/L, calculate the solubility product constant (Ksp)…
A: The solubility product constant (Ksp) represents the solubility of products at equilibrium for…
Q: On analysis, an equilibrium mixture for the reaction 2H2S(g) 2H2(g) + S2(g)was found to contain 1.0…
A: Equilibrium constant-It is defined as the ratio of the equilibrium concentration of the products…
Q: Write the equilibrium expression for Ca^2+ + 2Cl^- ⇌ CaCl2 with activity coefficients. pls answer…
A: activity = activity co-efficients * molality(or,concentration term)
Q: The solubility of Cd(OH)2 in water at a certain temperature is 2.0 × 10–5 mol/L, (i.e., x). The Ksp…
A: Given: The solubility of Cd(OH)2 in water at a certain temperature = 2.0 × 10-5 mol/L We have to…
Q: The equilibrium constant for the reaction 2NO(g)+02(g)-2NO2(g) was measured at various temperatures.…
A: Recall the given reaction 2NOg + O2g ↔2NO2 Here a plot of lnK vs 1T is given.And value of y…
Q: How the equilibrium constant K' of a reaction will change if the temperature rises from 25ºC to 37ºC…
A: The standard free energy change of the reaction, ΔG0 is equal to the difference between the free…
Q: Silver chromate is sparingly soluble in aqueous solutions. The Kp of Ag, CrO, is 1.12 × 10¬12, What…
A: Silver Chromate is a sparingly soluble salt. It's dissociation in an aqueous medium is written…
Q: Calculate Kp for each salt in an aqueous solution at 25 °C in which u = 0.0100 M. Use the table of K…
A: The solubility product constant (Ksp) represents the solubility of products at equilibrium for…
Q: The equilibrium constant for the reaction N2(g) + O2(g) 2 NO(g) is 1.7 x 10-1 at an elevated…
A: Let us consider a following reaction aA + bB = cC + dD then reaction Q is defined as Q = (pC)c x…
Q: Sufficient solid CaSO4 is added to enough water to produce a solution (if it all dissolves) that is…
A: a.) First we would write dissociation equation to CaSO4 and Calculate reaction quotient. b.) Then…
Q: In the gas-phase reaction A + B ⇋ C + 2 D, it was found that, when 2.00 mol A, 1.00 mol B, and 3.00…
A: A + B⇌ C + 2Dt=0 2mol 1mol 0 3molt=eqn 2-x 1-x 0+x…
Q: Lead chloride has limited aqueous solubility, based on the solubility product below. This Kp Value…
A: The reaction of PbCl2 can be represented as;…
Q: The rate of accumulation of salt in the tank is equal to: O the product of the concentration and the…
A: The concentration of the salt solution is given by: concentration of the salt solution = mass of…
Q: Write the expression for the equilibrium constant, Kc , for the following system: CaCO3(s) ⇄⇄…
A: Calcium carbonate is the salt of calcium which is the main component for the synthesis of pearls,…
Q: In the laboratory, a general chemistry student measured the pH of a 0.591 M aqueous solution of…
A: Aniline is a weak base , lets find its pOH pH +pOH = 14 (at 25oC) 9.305 + pOH = 14 pOH = 14 -…
Q: Consider the insoluble compound silver chloride, AgCl. The silver ion also forms a complex with…
A: The solubility equilibria is the equilibrium existing between a solid and its associated ions in the…
Q: ilver chromate is sparingly soluble in aqueous solutions. The Kp of Ag,CrO, is 1.12 x 10-12 /hat is…
A: We will. Calculate solubility
Q: O Define molar solubility and solubility. O The solubility product, Ksp of silver chloride, AgCl is…
A: Given solubility product of AgCl is 1.0×10-10 Molar mass of AgCl is 143.3 g/mol.
Q: 2. The solubility of calcium sulfate (CASO4) is found to be 0.67 g/L. Calculate the value of Ksp for…
A: Molar solubility is equal to the maximum amount of solute that can be dissolved in per liter of…
Q: Express the equilibrium constant for the following reaction: 2 CH2CI2 (g) + Cl2 (g) =2 CHCI3 + H2…
A:
Q: For the liquid-phase reaction A + 2B ⇄ C + 2D , it was found that when 2 mol A, 4 mol B and 2 mol C…
A: The expression for the calculation of equilibrium constant is given below:
Q: The generic metal A forms in an insoluble salt Ab(s) and a complex AC5(aq) The equilibrium…
A:
Q: 7. The Ksp for copper(I) sulfide, Cu2S(s), is 2.5 x 10-48. Cu2S (s) =2 Cu'(aq) + S²-(aq) (a) Write a…
A: The dissociation of copper(I) sulfide is given by: Cu2Ss ⇌ 2Cu+aq + S2-aq The value of the…
Q: Use the References to access impertant values if needed for this question. Write a balanced net…
A: Introduction: The chemical equation shows the reaction between the two or chemical species and their…
Q: The Ksp for a very insoluble salt is 4.2 * 10-47 at 298 K. What is ∆G° for the dissolution of the…
A: Ksp = 4.2 × 10-47 T = 298 K
Q: Be sure to answer all parts. Given that the solubility of PbCro, is s = 4.0 x 10 g/L, calculate the…
A: Given the solubility of PbCrO4 is s =4.0×10-5 gLwe are asked to calculate the solubility product…
Q: For the following heterogeneous (solubility) equilibrium reaction, Ag2CrO4(s) s 2 Ag* + CrO42- Which…
A: Ag2CrO4(s) <.....> 2Ag+ + CrO42- Solubility product constant (Ksp) tells the solubility of…
Q: For items 10-12. The decomposition of NH,Cl (s) at a given temperature of 548 K, the Kp = 0.01072.…
A:
Q: If 500.0 mLmL of 0.10 mol L−1 Ca2+mol L−1 Ca2+ is mixed with 500.0 mLmL of 0.10 mol L−1 SO42−mol L−1…
A: In a solution, when the amount of salt added it more than the solubility amount of the salt, the…
Q: A mixture consisting of 1.000 mol H2O(g) and 1.000 mol CO(g) is placed ina reaction vessel of volume…
A: Using the relationship of Kp and Kc, value of Kp can be determined.
Q: For the reaction below, formation salpies at 1 bar and standart entropies are given. Çe te…
A:
Q: What is the molar solubility of silver sulfate (Ag,SO,) in water? The solubility-product constant…
A: Silver sulfate is insoluble in water, which means it shows equilibrium between the dissociated ions…
Q: Which of the following would not be found in the Law of Mass Action (expression for K) for this…
A: K = [CH3COO-][H3O+] / [CH3COOH]
Q: 7. The solubility product value for copper(I) iodate, Cu(lO3)2, is 1.4 x 107 at 25°C. Calculate its…
A:
Q: In the gas phase reaction 2 A(g) + B(g) = 3 C (g) + 2 D (g), it was found that when 1.00 mol A, 2.00…
A:
Q: When 18.0 mL of a 4.67×104 M sodium sulfide solution is combined with 18.0 mL of a 4.42x104 M lead…
A: We have to tell whether precipitate will form or not
Q: A mixture consiting of 1.000 mol H2O(g) and 1.000 mol CO(g) is placed ina reaction vessel of volume…
A: Initial concentration of CO and H2O and also the concentration of CO2 at equilibrium can be…
Q: 6.5 g of thallium (I) chloride, TICI, is added to 750 mL of water at room temperature to determine…
A: Given: Mass of TlCl remaining after evaporation = 1.20 g. Volume of solution evaporated = 400 mL =…
Q: A student measures the S2- concentration in a saturated aqueous solution of cobalt(II) sulfide to be…
A:
Q: For the following heterogeneous (solubility) equilibrium reaction, Ag2CrO4(s) s 2 Ag* + CrO42- Which…
A: Solid Ag2CrO4 is partially soluble in aqueous solution and forming equilibrium with aqueous Ag+ and…
Q: Consider a body of water in equilibrium with solid calcium sulfate, CaSO4, for which Ksp = 3.0 *…
A:
Q: The solubility of silver phosphate, Ag3PO4, at 25°C is 1.59 × 10–5 mol/L. What is the Ksp for the…
A: Given that The solubility of silver phosphate, Ag3PO4, at 25°C = 1.59 × 10–5 mol/L. To find: Ksp…
Q: The solubility product constant, Ksp, for barium sulfate, BaSO4, is 1.1 x 10-10. Compare the…
A: The solubility product constant, Ksp, for barium sulfate, BaSO4 = 1.1×10–10 Concentration of sodium…
Q: Consider the dissolution reactions below for the simple salts NaCl (s). AgCl (s), and CaCl2 (s). The…
A: “Since you have posted a question with multiple sub-parts, we will solve the first three subparts…
Q: For items 10-12. The decomposition of NH4CI (s) at a given temperature of 548 K, the Kp = 0.01072.…
A:
Step by step
Solved in 2 steps with 2 images

- The concentration of calcium carbonate in a sample of water saturated with the solid was found to be 7.00 x 10-5 moldm-3 at 200C. What is the solubility product of calcium carbonate at this temperature?In an experiment to calculate the solubility product (Ksp) of barium nitrate (Ba(NO3)2), an excess amount of Ba(NO3)2 was added to 2 liters of water at 25oC until the solution is saturated. Because Ba(NO3)2 is only slightly soluble in water, the excess Ba(NO3)2 was filtered out to get a solid-free mixture. The proponents of the experiment thought about using the concept of colligative properties, specifically boiling point elevation, to determine Ksp. It was observed in their experiments that the solution boils at 100.15 K. Barium nitrate dissociates via the process: Ba(NO3)2(s) ⇄ Ba2+(aq) + 2NO?-3(?q) Ksp = [Ba2+][NO3−]2 Calculate the following if Kb,water = 0.51 K-kg/mol: a) Amount of Ba(NO3)2 dissolved in grams. Molar mass of Ba(NO3)2 = 261.3 g/mol b) Solubility product, Ksp c) Vapor pressure of the solution in kPaIn an experiment to calculate the solubility product (Ksp) of barium nitrate (Ba(NO3)2), an excess amount of Ba(NO3)2 was added to 2 liters of water at 25oC until the solution is saturated. Because Ba(NO3)2 is only slightly soluble in water, the excess Ba(NO3)2 was filtered out to get a solid-free mixture. The proponents of the experiment thought about using the concept of colligative properties, specifically boiling point elevation, to determine Ksp. It was observed in their experiments that the solution boils at 100.15 K. Barium nitrate dissociates via the process: ?a(??3)2(?) ⇄ ??2+(?q) + 2??-3(?q) ??p = [??2+][??3−]2 Calculate the following if Kb,water = 0.51 K-kg/mol: a) Amount of Ba(NO3)2 dissolved in grams. Molar mass of Ba(NO3)2 = 261.3 g/mol b) Solubility product, Ksp c) Vapor pressure of the solution in kPa
- In an experiment to calculate the solubility product (Ksp) of barium nitrate (Ba(NO3)2), an excess amount of Ba(NO3)2 was added to 2 liters of water at 25oC until the solution is saturated. Because Ba(NO3)2 is only slightly soluble in water, the excess Ba(NO3)2 was filtered out to get a solid-free mixture. The proponents of the experiment thought about using the concept of colligative properties, specifically boiling point elevation, to determine Ksp. It was observed in their experiments that the solution boils at 100.15oC. Barium nitrate dissociates via the process: Ba(NO3)2(s) ⇄ Ba2+(aq) + 2NO?-3(?q) Ksp = [Ba2+][NO3−]2 Calculate the following if Kb,water = 0.51 K-kg/mol: a) Amount of Ba(NO3)2 dissolved in grams. Molar mass of Ba(NO3)2 = 261.3 g/mol b) Solubility product, Ksp c) Vapor pressure of the solution in kPaOne mixes aqueous NaCl with aqueous AgNO3. NaCl. AgNO3and NaNO3all have appreciable solubility in water. AgCl has a solubility product constant Ksp of 1.6 x 10-10. If one mixes the NaCl solution with the AgNO3solution, one would suspect: a) no precipitate will form b) AgCl precipitate will form c) NaNO3 precipitate will form d) both AgCl and NaNO3 precipitates will formCalculate the solubility at 25°C of CuBr in pure water and in a 0.0100M CoBr2 solution. You'll find Ksp data in the ALEKS Data tab. Round both of your answers to 2 significant digits. solubility in pure water: gL solubility in 0.0100 M CoBr2solution: gL
- Write an appropriate expression for the solubility product of the sparingly soluble compound Ca(OH)2 Given the solubility product, Ksp, of Ca(OH)2 is 5.3x10-5M3 at 25oC, calculate its solubility at this temperature in Water 25 M CaCl2 solutionThe following evidence was obtained from an experiment to determine the solubility of calcium chloride at room temperature. A sample of saturated calcium chloride solution was evaporated to dryness, and the mass of solid residue was measured.EvidenceVolume of solution (mL) = 15.0Mass of empty beaker (g) = 90.54Mass of beaker and residue (g) = 101.36The solubility of calcium chloride is g/100 mLIn the interface between sediment and water around a deep lake bottom, dissolved oxygen (DO) is usually low. Under such a condition, iron and sulfur typically exist in their reduction forms (i.e. Fe2+ and S2-). Fe2+ and S2- can readily react with each other to produce ferrous sulfide (FeS), which is a black solid substance and finally settles down into the sediments at the lake bottom. The solubility product constant (i.e. equilibrium constant) of FeS is 8 x 10-9. At a chemical equilibrium state, what is the concentration of S2- in the water nearby the sediments if Fe2+ concentration in water is 10 - 4 M.
- Lactic acid (CH3 - CH(OH) - COOH) is a weak acid and therefore a weak electrolyte.found in "cut milk". The freezing point of an aqueous solution 0.01 m of lactic acid is 0.0206 ºC. Knowing that in aqueous solution the following equilibrium occurs:CH3 -CH(OH) -COOH(ac) = CH3 -CH(OH) -COO(-)(ac) + H(+)(ac)and that, therefore, there are three species (solutes) in solution, calculate their percentage of ionization.Lactic acid (CH3 - CH(OH) - COOH) is a weak acid and therefore a weak electrolyte. found in "cut milk". The freezing point of an aqueous solution 0.01 m of lactic acid is 0.0206 ºC. Knowing that in aqueous solution the following equilibrium occurs: CH3 -CH(OH) -COOH(ac) = CH3 -CH(OH) -COO(-)(ac) + H(+)(ac) and that, therefore, there are three species (solutes) in solution, calculate their percentage of ionization.On analysis, an equilibrium mixture for the reaction 2H2S(g) 2H2(g) + S2(g)was found to contain 1.0 mol H2S, 4.0 mol H2, and 0.80 mol S2 in a 4.0L vessel.Calculate the equilibrium constant, Kc, for this reaction.

