Could you give me a level 4 example of a prediction as a Hypothesis (in the form of an If... then... statement) and provide reasoning based on grade 12 (SCH4U) knowledge of electron configurations about the lab attached.
Could you give me a level 4 example of a prediction as a Hypothesis (in the form of an If... then... statement) and provide reasoning based on grade 12 (SCH4U) knowledge of electron configurations about the lab attached.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter34: Particle Size Determination
Section: Chapter Questions
Problem 34.15QAP
Related questions
Question
Could you give me a level 4 example of a prediction as a Hypothesis (in the form of an If... then... statement) and provide reasoning based on grade 12 (SCH4U) knowledge of electron configurations about the lab attached.
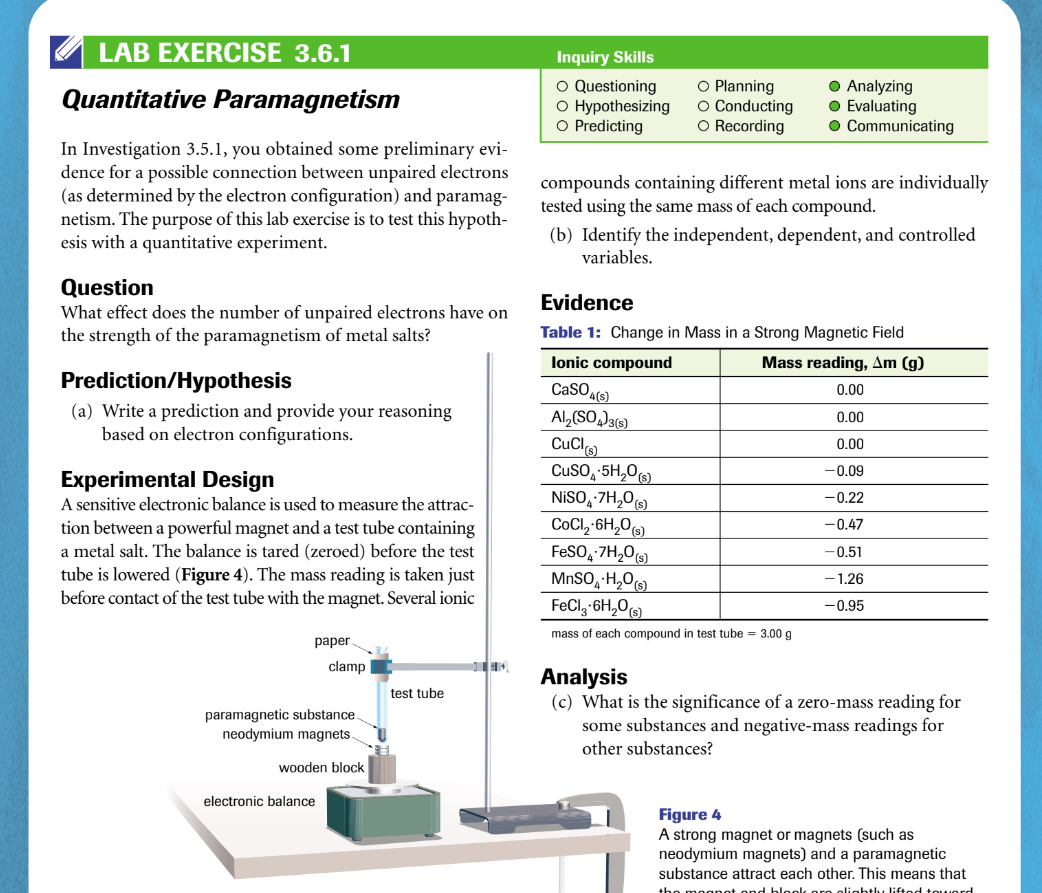
Transcribed Image Text:LAB EXERCISE 3.6.1
Quantitative Paramagnetism
In Investigation 3.5.1, you obtained some preliminary evi-
dence for a possible connection between unpaired electrons
(as determined by the electron configuration) and paramag-
netism. The purpose of this lab exercise is to test this hypoth-
esis with a quantitative experiment.
Question
What effect does the number of unpaired electrons have on
the strength of the paramagnetism of metal salts?
Prediction/Hypothesis
(a) Write a prediction and provide your reasoning
based on electron configurations.
Experimental Design
A sensitive electronic balance is used to measure the attrac-
tion between a powerful magnet and a test tube containing
a metal salt. The balance is tared (zeroed) before the test
tube is lowered (Figure 4). The mass reading is taken just
before contact of the test tube with the magnet. Several ionic
paper.
clamp
paramagnetic substance.
neodymium magnets.
wooden block
electronic balance
test tube
H
Inquiry Skills
O Questioning
O Hypothesizing
O Predicting
O Planning
O Conducting
O Recording
compounds containing different metal ions are individually
tested using the same mass of each compound.
● Analyzing
● Evaluating
O Communicating
(b) Identify the independent, dependent, and controlled
variables.
CuSO4.5H₂O(s)
NiSO4.7H₂O(s)
CoCl₂-6H₂O(s)
FeSO4.7H₂20 (s)
Evidence
Table 1: Change in Mass in a Strong Magnetic Field
Ionic compound
Mass reading, Am (g)
0.00
CaSO4(s)
Al₂(SO4)3(s)
0.00
CuCl(s)
0.00
-0.09
-0.22
-0.47
-0.51
-1.26
-0.95
MnSO4 H₂O(s)
FeCl3 6H₂O(s)
mass of each compound in test tube = 3.00 g
Analysis
(c) What is the significance of a zero-mass reading for
some substances and negative-mass readings for
other substances?
Figure 4
A strong magnet or magnets (such as
neodymium magnets) and a paramagnetic
substance attract each other. This means that
the magnet and block ora olightly lifted toward
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning