Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 6RQ: In terms of Raoults law, distinguish between an ideal liquid-liquid solution and a nonideal...
Related questions
Question
Could you please write me a brief summary about the results that I have and it's about the standardization of sodium hydroxide
thank you
Transcribed Image Text:00
5.
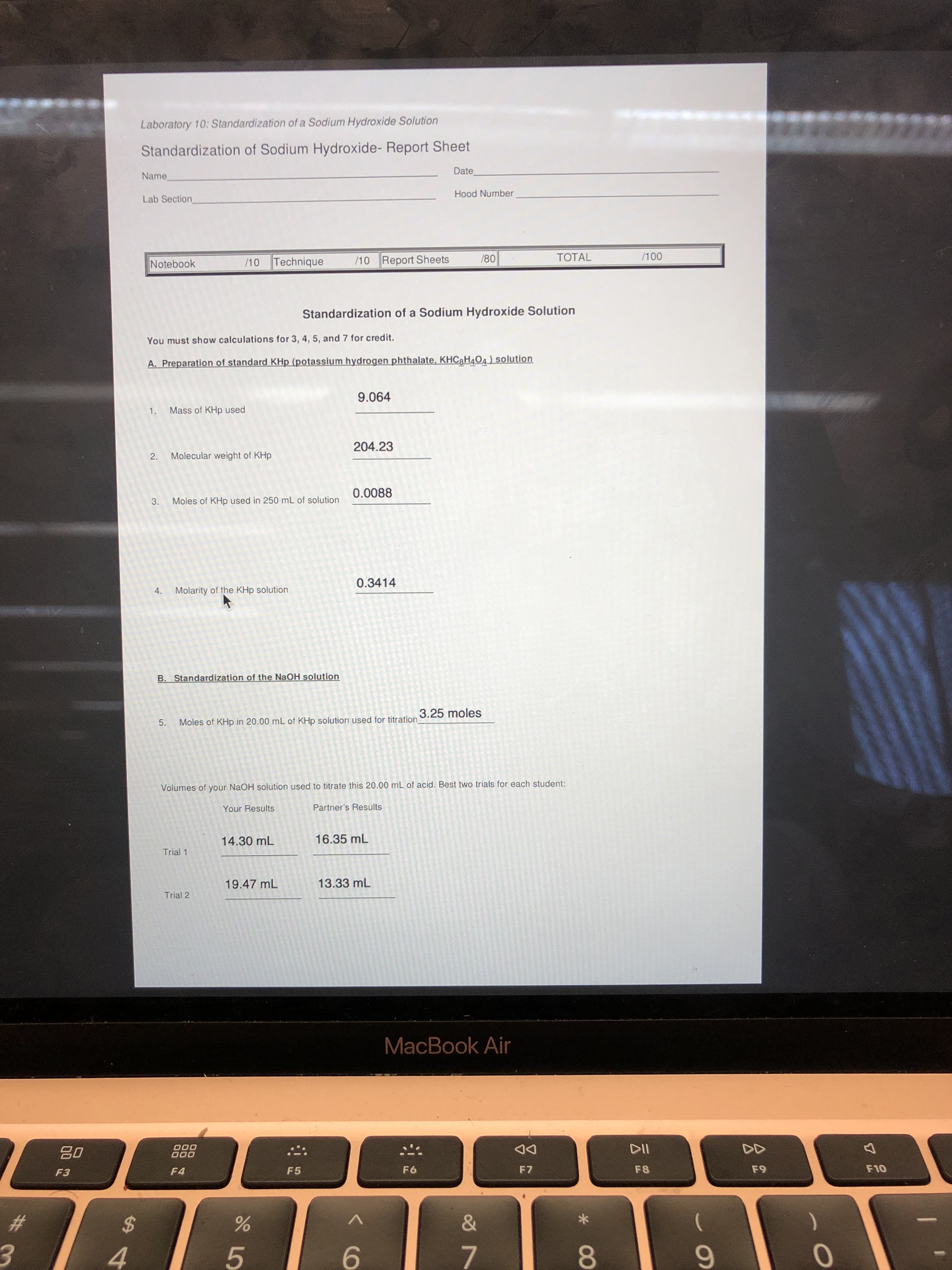
Laboratory 10: Standardization of a Sodium Hydroxide Solution
Standardization of Sodium Hydroxide- Report Sheet
Name
Date
Lab Section
Hood Number
Notebook
/10 Technique
/10 Report Sheets
/80
TOTAL
Standardization of a Sodium Hydroxide Solution
You must show calculations for 3, 4, 5, and 7 for credit.
A. Preparation of standard KHp (potassium hydrogen phthalate, KHC3H4O4) solution
9.064
1. Mass of KHp used
204.23
2. Molecular weight of KHp
0.0088
3. Moles of KHp used in 250 mL of solution
0.3414
4.
Molarity of the KHp solution
B. Standardization of the NaOH solution
3.25 moles
Moles of KHp in 20.00 mL of KHp solution used for titration
Volumes of your NaOH solution used to titrate this 20.00 mL of acid. Best two trials for each student:
Your Results
Partner's Results
14.30 mL
16.35 mL
Trial 1
19.47 mL
13.33 mL
Trial 2
MacBook Air
000
000
02
F3
DD
F7
F4
F5
%23
24
4.
5.
6.
7.
6

Transcribed Image Text:14.84 mL
Best Value
6. Explain how you arrived at your best value.
0.22
7.
Molarity of your NaOH solution:
6. The purposse of executing trials in quantitative analysis is to find the deviation in the results. The lesser the deviation between
the values obtained in trials, the precise the result is.In the case of my result, the difference between the results obtained in Trial 1
and Trial 2 is16.35mL - 13.33mL = 3.02 mL. But in the case of my partner's result, the difference between the results obtained in
Trial 1 and Trial 2 is19.47mL - 14.30 mL = 5.17 mL.There appears relatively large deviation in my partner's result. My result is
better than my partner's result. The average of my result = 16.35mL + 13.33mL 216.35mL+ 13.33m / 2 = 29.68mL /2 = 14.84 mL
= 14.84 mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
