Course: College Chemistry Course code: CHM 141 Complete the table that follows, indicate changes in moles by entering I, D, N or? in the table (In =Increase, D =Decrease, N= No change? = Insufficient information to determine.) and if Equilibrium shift write Left, Right or No Change. Please fill in all the blank tables. Do not Incomplete. a b C d Using Le Châtelier's Principle, predict the effect of changing various conditions on the following equilibrium system: 2 CO2(g) + N2(g) + heat = C₂N2(g) + O2(g) Equilibrium shift (left, right, no change) Amount Amount Amount Amount CO₂ N₂ C₂N₂ added Other catalyst added added temperature increased
Course: College Chemistry Course code: CHM 141 Complete the table that follows, indicate changes in moles by entering I, D, N or? in the table (In =Increase, D =Decrease, N= No change? = Insufficient information to determine.) and if Equilibrium shift write Left, Right or No Change. Please fill in all the blank tables. Do not Incomplete. a b C d Using Le Châtelier's Principle, predict the effect of changing various conditions on the following equilibrium system: 2 CO2(g) + N2(g) + heat = C₂N2(g) + O2(g) Equilibrium shift (left, right, no change) Amount Amount Amount Amount CO₂ N₂ C₂N₂ added Other catalyst added added temperature increased
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.25QAP
Related questions
Question
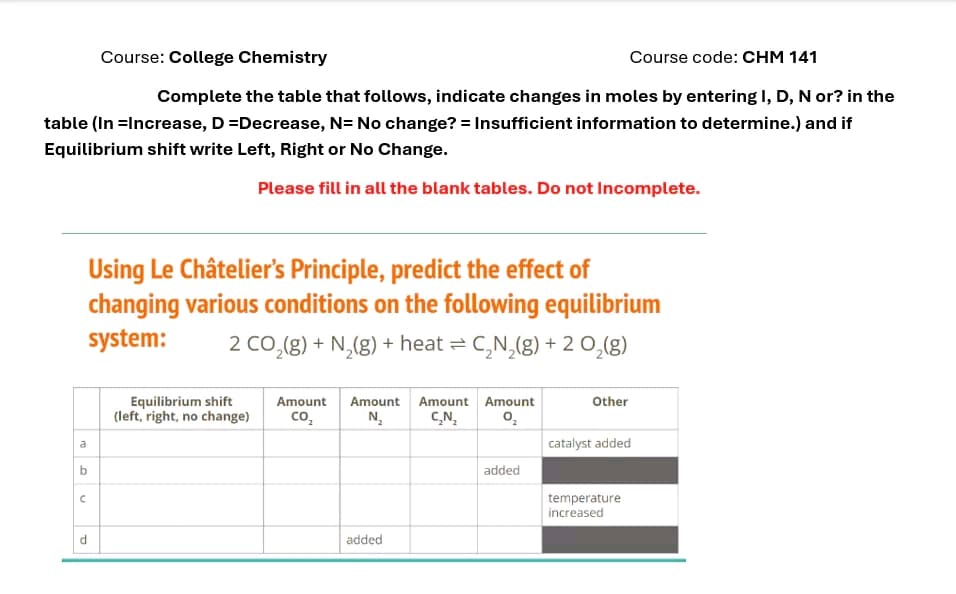
Transcribed Image Text:Course: College Chemistry
Course code: CHM 141
Complete the table that follows, indicate changes in moles by entering I, D, N or? in the
table (In =Increase, D =Decrease, N= No change? = Insufficient information to determine.) and if
Equilibrium shift write Left, Right or No Change.
Please fill in all the blank tables. Do not Incomplete.
a
b
C
d
Using Le Châtelier's Principle, predict the effect of
changing various conditions on the following equilibrium
system:
2 CO2(g) + N2(g) + heat = C₂N2(g) + O2(g)
Equilibrium shift
(left, right, no change)
Amount Amount Amount Amount
CO₂
N₂
C₂N₂
added
Other
catalyst added
added
temperature
increased
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

