What is an appropriate stepwise synthesis for the following synthesis that uses 1-propanol as the only source of carbon and any other reagents as needed? CH3CH2CH2OH CH3CH2CH2CHCOCH2CH2CH3 CH3 O A. 1-propanol reacts with PCC/CH2Cl2 to yield an intermediate that reacts with LDA to yield intermediate X, which has a resonance form Y. 1-propanol also reacts with PCl3 to yield an intermediate Z that reacts with intermediate X, which undergoes the following series of reactions to yield the product: 1. Na2Cr2O7/H2SO4/H2O; 2. Excess CH3CH2CH2OH/H2SO4. OB. 1-propanol reacts with PCC/CH2Cl2, then the product reacts with LDA to produce intermediate X, which has resonance forms. 1-propanol reacts with PCl3, then the product reacts with intermediate X to yield intermediate Y. Intermediate Y reacts with NaBH4/CH3OH to yield the product. SUPPORT OC. 1-propanol reacts with PCl3 to yield intermediate X. 1-propanol also undergoes the following reaction sequence: 1. Na2Cr2O7/H2SO4/H2O; 2. excess CH3CH2CH2OH/H2SO4; 3. LDA; 4. Intermediate X. This yields the product.
What is an appropriate stepwise synthesis for the following synthesis that uses 1-propanol as the only source of carbon and any other reagents as needed? CH3CH2CH2OH CH3CH2CH2CHCOCH2CH2CH3 CH3 O A. 1-propanol reacts with PCC/CH2Cl2 to yield an intermediate that reacts with LDA to yield intermediate X, which has a resonance form Y. 1-propanol also reacts with PCl3 to yield an intermediate Z that reacts with intermediate X, which undergoes the following series of reactions to yield the product: 1. Na2Cr2O7/H2SO4/H2O; 2. Excess CH3CH2CH2OH/H2SO4. OB. 1-propanol reacts with PCC/CH2Cl2, then the product reacts with LDA to produce intermediate X, which has resonance forms. 1-propanol reacts with PCl3, then the product reacts with intermediate X to yield intermediate Y. Intermediate Y reacts with NaBH4/CH3OH to yield the product. SUPPORT OC. 1-propanol reacts with PCl3 to yield intermediate X. 1-propanol also undergoes the following reaction sequence: 1. Na2Cr2O7/H2SO4/H2O; 2. excess CH3CH2CH2OH/H2SO4; 3. LDA; 4. Intermediate X. This yields the product.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter23: Addition To A Carbonyl
Section: Chapter Questions
Problem 42CTQ
Related questions
Question
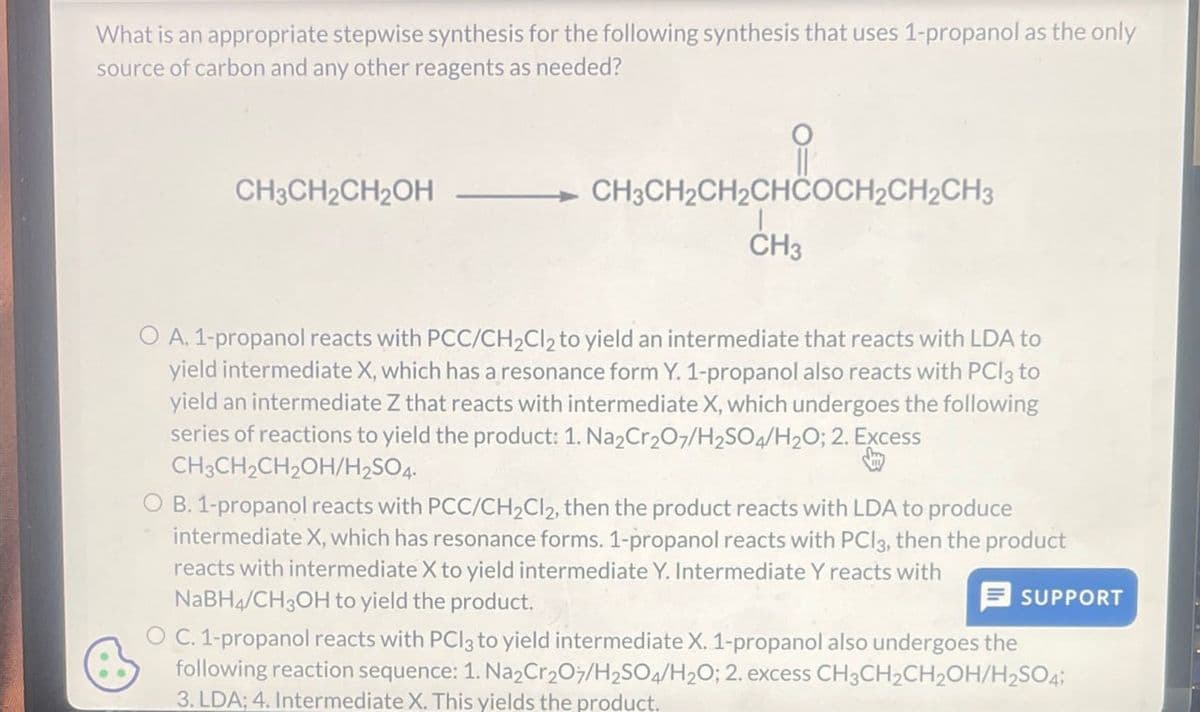
Transcribed Image Text:What is an appropriate stepwise synthesis for the following synthesis that uses 1-propanol as the only
source of carbon and any other reagents as needed?
CH3CH2CH2OH
CH3CH2CH2CHCOCH2CH2CH3
CH3
O A. 1-propanol reacts with PCC/CH2Cl2 to yield an intermediate that reacts with LDA to
yield intermediate X, which has a resonance form Y. 1-propanol also reacts with PCl3 to
yield an intermediate Z that reacts with intermediate X, which undergoes the following
series of reactions to yield the product: 1. Na2Cr2O7/H2SO4/H2O; 2. Excess
CH3CH2CH2OH/H2SO4.
OB. 1-propanol reacts with PCC/CH2Cl2, then the product reacts with LDA to produce
intermediate X, which has resonance forms. 1-propanol reacts with PCl3, then the product
reacts with intermediate X to yield intermediate Y. Intermediate Y reacts with
NaBH4/CH3OH to yield the product.
SUPPORT
OC. 1-propanol reacts with PCl3 to yield intermediate X. 1-propanol also undergoes the
following reaction sequence: 1. Na2Cr2O7/H2SO4/H2O; 2. excess CH3CH2CH2OH/H2SO4;
3. LDA; 4. Intermediate X. This yields the product.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning