CuBr. Cui63.5/223k100=28.4%omg Br: 19.9 *2-159.8/223.3x100 - 71.6%img Na OH Na: 25.0/40x (60-57.5% ng 0.16.0/40x160:40% mg Hil.0/40x100 :2.5% mg I4;1,0.8=8/68.1x100-11.77,4g 3:32.1/68.1x1G0 = 47.1% ng
CuBr. Cui63.5/223k100=28.4%omg Br: 19.9 *2-159.8/223.3x100 - 71.6%img Na OH Na: 25.0/40x (60-57.5% ng 0.16.0/40x160:40% mg Hil.0/40x100 :2.5% mg I4;1,0.8=8/68.1x100-11.77,4g 3:32.1/68.1x1G0 = 47.1% ng
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section3.10: Solution Concentration: Molarity
Problem 3.23E
Related questions
Question
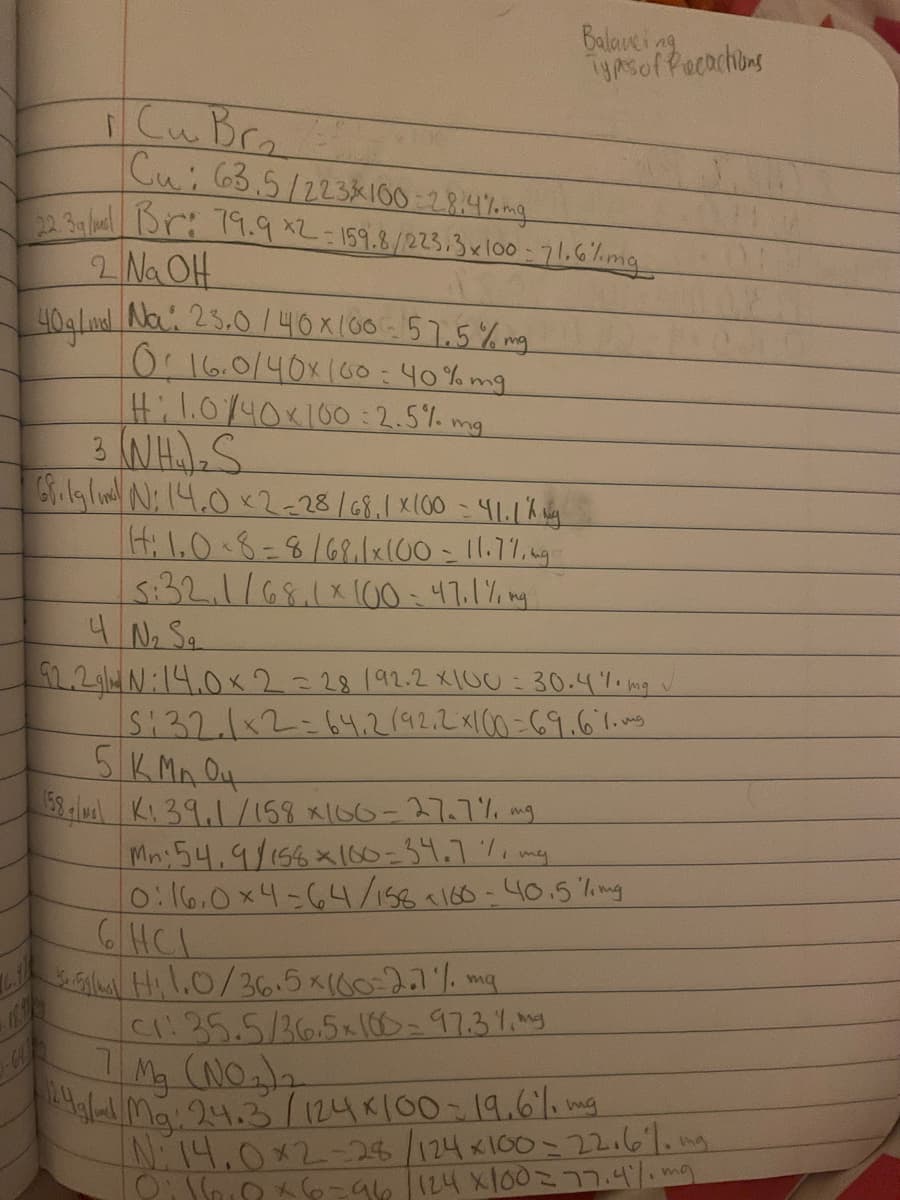
Transcribed Image Text:Balavei
TyAsoffecochions
Cu Br.
Cui 63.5/22381I00 -2R.4%mg
23a aBr: 19.9 *2-159.8/223.3xlo0 - 71.6%mg
2 Na OH
40alud Na: 25.0/40x100:57.5% mg
0.16.0/40x160:40% mg
Hi1.0/40x100:2.5%. mg
3 NH)S
(4:1,0.8=8/68lx(00-11.7%,ng
5:32.1/68.111I00:47.1% ng
4 No Sa
2.2gl4 N:14.0x2-28192.2 X1U : 30.4%i mg v
5:32.1x2=64.2142.2 x1C0 =69.6.1.ag
5K MA O4
58 lul Ki 39,1/158 x100-27.7%, mg
Mn:54,9/156x160=34.7%, my
0:16.0x4-64/158160-40.5 hmng
6HCI
SGal Hil.0/36.5x(60-2.7% mg
C1:35.5/36.5x100=97.3%. my
Mg (NO)2
Ma 24.3 /124K100-19.6lo
N:14.0x2-24/124x100=22.6%. mg
mg
Expert Solution
Step 1
Note: As per our guidelines, we are supposed to answer only the first three questions (i.e. 1 to 3 ). Kindly repost other questions separately.
Standard data:
- The molar mass of CuBr2 = 223.3 g/mol
- The molar mass of NaOH = 40 g/mol
- The molar mass of (NH4)2S = 68.1 g/mol
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning