cylinder, water, and silver object had a total mass of 56.89 g. Calculate the density of silver in g/cm3. Express your answer to two significant figures. i ! g/cm3 eTextbook and Media Hint Assistance Used Save for Later Last saved 1 hour ago. Attempts: 5 of 15 used Submit Answer Saved work will be auto-submitted on the due date. Auto- submission can take up to 10 minutes. 四山TA SEP 19 ... MacBook Air DD 888 F7 F8 F9 F10 F5 F6 F4 F2 F3 # $ & @ 2 3 4 6 7 8.
cylinder, water, and silver object had a total mass of 56.89 g. Calculate the density of silver in g/cm3. Express your answer to two significant figures. i ! g/cm3 eTextbook and Media Hint Assistance Used Save for Later Last saved 1 hour ago. Attempts: 5 of 15 used Submit Answer Saved work will be auto-submitted on the due date. Auto- submission can take up to 10 minutes. 四山TA SEP 19 ... MacBook Air DD 888 F7 F8 F9 F10 F5 F6 F4 F2 F3 # $ & @ 2 3 4 6 7 8.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 56RGQ: You are asked to identify an unknown liquid that is known to be one of the liquids listed below. You...
Related questions
Question
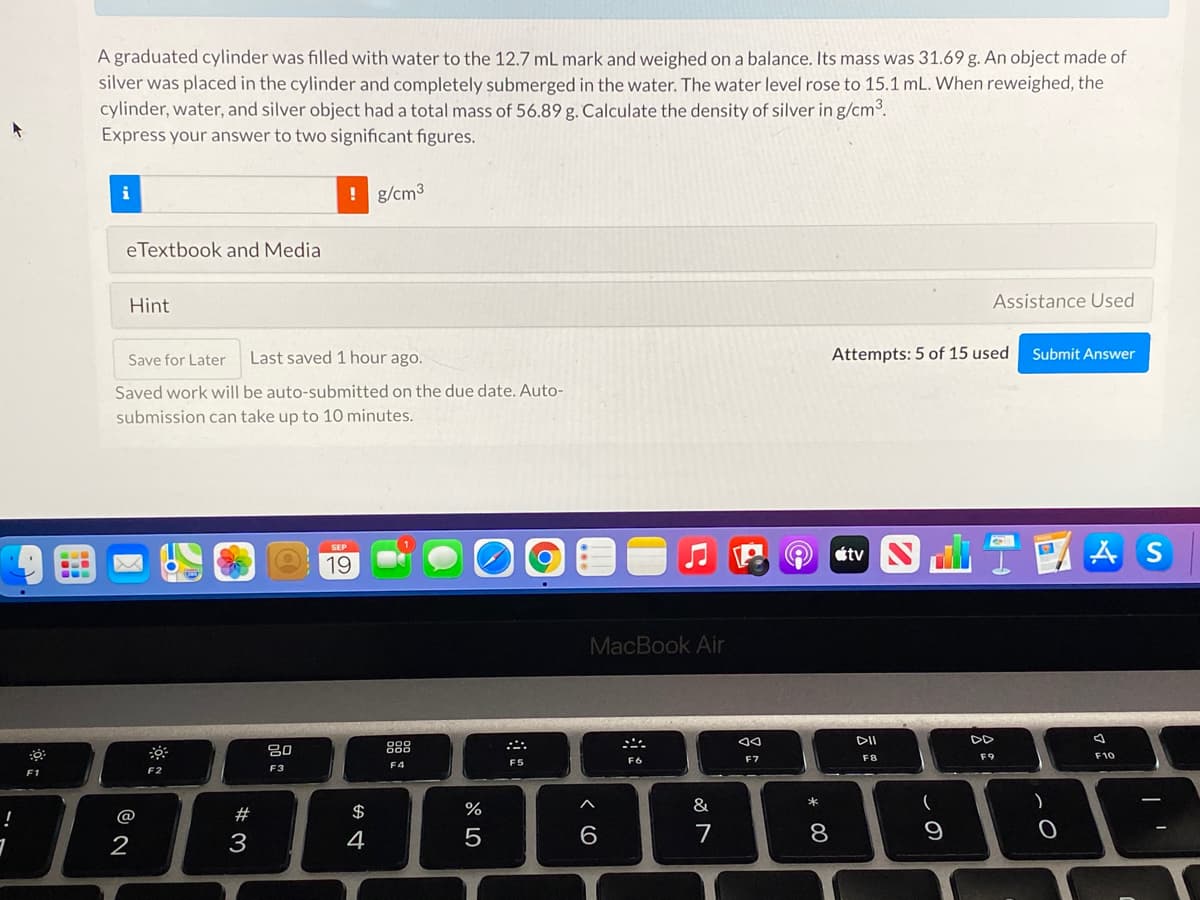
Transcribed Image Text:A graduated cylinder was filled with water to the 12.7 mL mark and weighed on a balance. Its mass was 31.69 g. An object made of
silver was placed in the cylinder and completely submerged in the water. The water level rose to 15.1 mL. When reweighed, the
cylinder, water, and silver object had a total mass of 56.89 g. Calculate the density of silver in g/cm3.
Express your answer to two significant figures.
i
! g/cm3
eTextbook and Media
Hint
Assistance Used
Save for Later
Last saved 1 hour ago.
Attempts: 5 of 15 used
Submit Answer
Saved work will be auto-submitted on the due date. Auto-
submission can take up to 10 minutes.
回TA
S
SEP
19
MacBook Air
DII
DD
吕0
888
F6
E7
F8
F9
F10
F4
F5
F2
F3
F1
!
@
$
%
2
3
4
6.
7
8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning