Da IXL- Write varia X Da IXL-Simplify ve X D0 IXL - Write varia K RC Gram to gra X Da IXL-Arc i web.kamihq.com/web/viewer.html?state%=%7B"ids"%3A%5B"1wajhTUytl3ZW_uZZQ2XqYnjACP40NAQX"%5D marks Maya Paniagua - Cl. nt Edu O e A My Drive RC Gram to gram stoich.pdf Chemistry Gram to Gram Stoichiometry Show all work for credit!! Balance the following equations and then perform the requested calculations 1. N2 + H2 → NH3 How many grams of ammonia are produced when 26.25 grams of hydrogen gas react with nitrogen gas?
Da IXL- Write varia X Da IXL-Simplify ve X D0 IXL - Write varia K RC Gram to gra X Da IXL-Arc i web.kamihq.com/web/viewer.html?state%=%7B"ids"%3A%5B"1wajhTUytl3ZW_uZZQ2XqYnjACP40NAQX"%5D marks Maya Paniagua - Cl. nt Edu O e A My Drive RC Gram to gram stoich.pdf Chemistry Gram to Gram Stoichiometry Show all work for credit!! Balance the following equations and then perform the requested calculations 1. N2 + H2 → NH3 How many grams of ammonia are produced when 26.25 grams of hydrogen gas react with nitrogen gas?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter13: The Chemistry Of Solutes And Solutions
Section13.7: Colligative Properties Of Solutions
Problem 13.16E: Suppose that you are closing a cabin in the north woods for the winter and you do not want the water...
Related questions
Question
Transcribed Image Text:Da IXL - Write varia X
Da IXL - Simplify ve x
0. IXL - Write varia X
K RC Gram to grai X
EL IXL - ArcI
i web.kamihq.com/web/viewer.html?state%=%7B"ids"%3A%5B"1wajhTUytl3ZW_uzZQ2XqYnjACP40NAQX"%5D
marks
E Maya Paniagua - Cl.
nt Edu O
A My Drive ►
RC Gram to gram stoich.pdf
Chemistry
Gram to Gram Stoichiometry
Show all work for credit!!
Balance the following equations and then perform the requested calculations
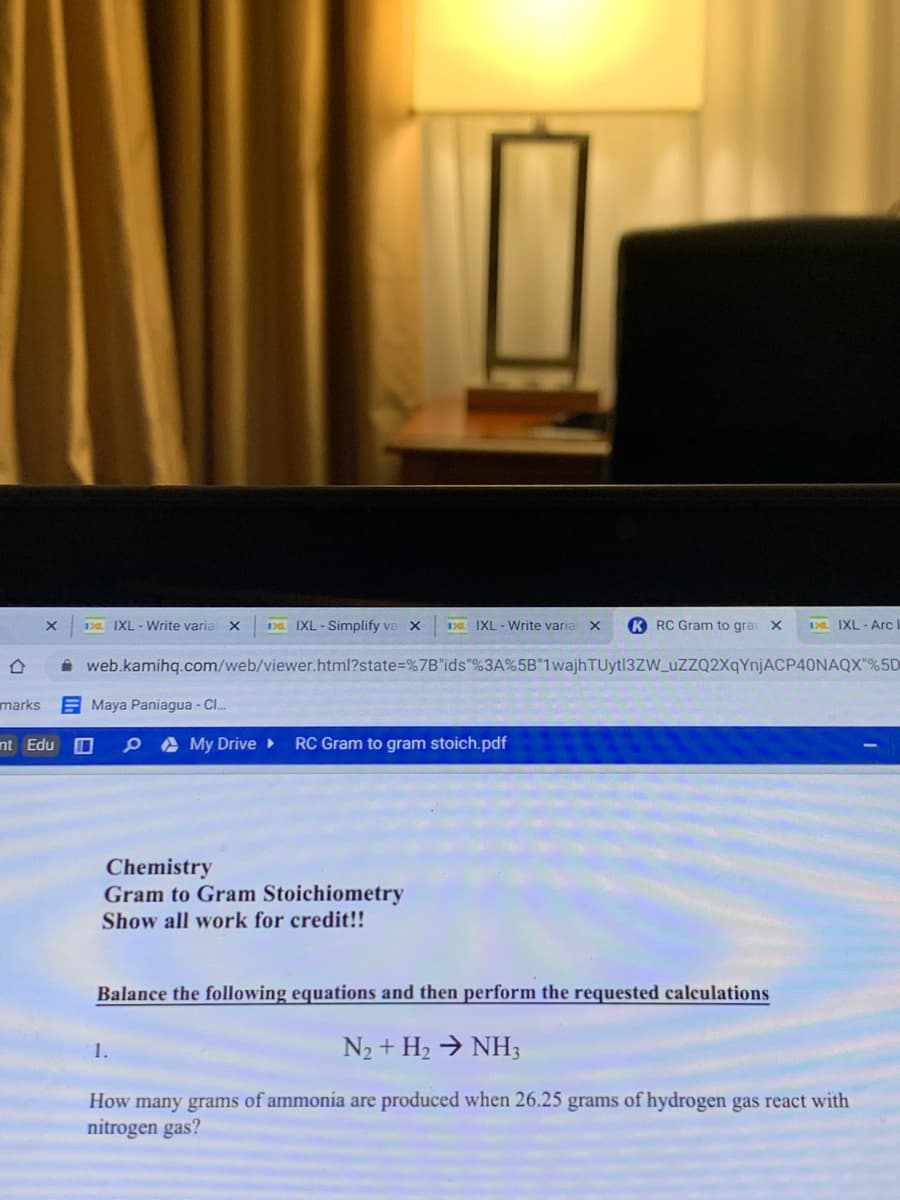
1.
N2 + H2 → NH3
How many grams of ammonia are produced when 26.25 grams of hydrogen gas react with
nitrogen gas?
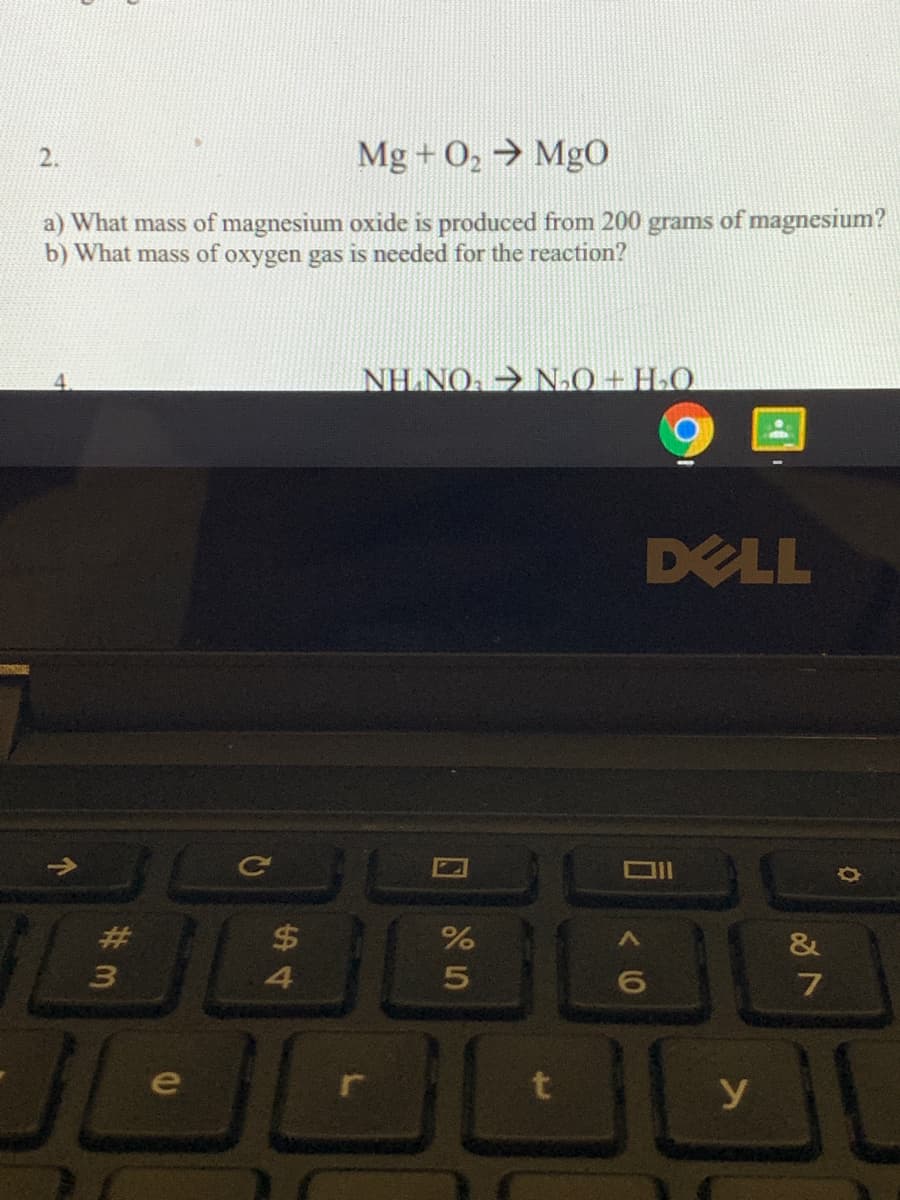
Transcribed Image Text:2.
Mg + O, → MgO
a) What mass of magnesium oxide is produced from 200 grams of magnesium?
b) What mass of oxygen gas is needed for the reaction?
OH+ON EF ON HN
DELL
23
&
4.
e
y
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning