(d) The analytical information for compound (1) is provided below. Molecular Formula IR spectrum 'H NMR spectrum CHBO₂ A strong band at 1716 cm¹ 11.7 ppm (singlet - Integral 1H); 2.38 ppm (quartet - integral 2H); 1.16 ppm (triplet - integral 3H); (1) Suggest proton environments for the three resonances in the ¹H NMR spectrum, (i) Account for the wavenumber of the IR band, (ill) Deduce the structure of compound (I) using all the analytical data, and your answers to part (I).
(d) The analytical information for compound (1) is provided below. Molecular Formula IR spectrum 'H NMR spectrum CHBO₂ A strong band at 1716 cm¹ 11.7 ppm (singlet - Integral 1H); 2.38 ppm (quartet - integral 2H); 1.16 ppm (triplet - integral 3H); (1) Suggest proton environments for the three resonances in the ¹H NMR spectrum, (i) Account for the wavenumber of the IR band, (ill) Deduce the structure of compound (I) using all the analytical data, and your answers to part (I).
Chapter12: Structure Determination: Mass Spectrometry And Infrared Spectroscopy
Section12.SE: Something Extra
Problem 50AP
Related questions
Question
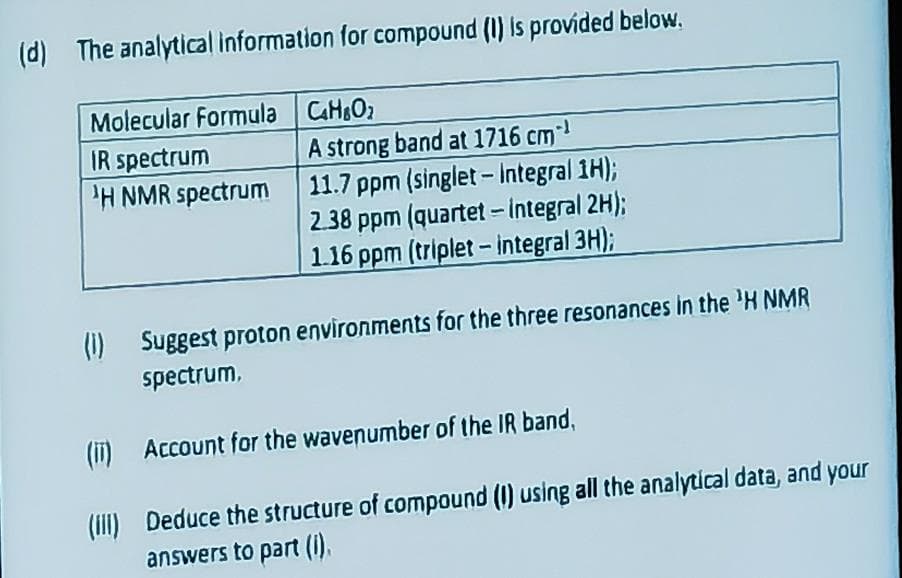
Transcribed Image Text:(d) The analytical information for compound (1) is provided below.
Molecular Formula
IR spectrum
'H NMR spectrum
C4HO₂
A strong band at 1716 cm¹
11.7 ppm (singlet - Integral 1H);
2.38 ppm (quartet - integral 2H);
1.16 ppm (triplet - integral 3H);
(1) Suggest proton environments for the three resonances in the ¹H NMR
spectrum,
(i)
Account for the wavenumber of the IR band,
(III) Deduce the structure of compound (I) using all the analytical data, and your
answers to part (I).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT