D. None of the above 11. A 315mL of water was added to 2100 mL of 19M NaCI solution. What is the new concentration of the solution? (MW: Na 23 g/mol, Cl 35g/mol) A. 2.85 M В. 2.48 M C. 18.60 M D. 19.0 M %3D 12. What is the molarity of a solution in which 150g of Cao are dissolved in 1900ml of solution? (MW: Ca 40g/mol, 0=16g/mol) A. 1.41 mol/L B. 0.0014 mol/L C. 0.0014 M D. 1.41 m 13. What is the normality of a solution containing 90g of HCI in 2600ml of solution? (MW: H 1g/mol, Cl=35g/mol) A. 0.00048 eq/mol B. 0.48 eq/mol C. 0.00096 eq/mol D. 0.96 eq/mol 14. Find the molality of a solution made by adding 8g of NaOCI to 5000g of water. (MW: Na 23 g/mol, 0=16 g/mol, Cl=35g/mol) A. 0.000021 m B. 0.02 m C. 0.1081 m D. 0.001081 m 15. 500g of Al:S is dissolved in H20 to make 15L of solution What is its normality? (MW: Al=27g/mol, S=32g/mol, H=lg/mol, O=16g/mol) 0.11 N B. 0.07 N C. 0.22 N D 0.04 N
D. None of the above 11. A 315mL of water was added to 2100 mL of 19M NaCI solution. What is the new concentration of the solution? (MW: Na 23 g/mol, Cl 35g/mol) A. 2.85 M В. 2.48 M C. 18.60 M D. 19.0 M %3D 12. What is the molarity of a solution in which 150g of Cao are dissolved in 1900ml of solution? (MW: Ca 40g/mol, 0=16g/mol) A. 1.41 mol/L B. 0.0014 mol/L C. 0.0014 M D. 1.41 m 13. What is the normality of a solution containing 90g of HCI in 2600ml of solution? (MW: H 1g/mol, Cl=35g/mol) A. 0.00048 eq/mol B. 0.48 eq/mol C. 0.00096 eq/mol D. 0.96 eq/mol 14. Find the molality of a solution made by adding 8g of NaOCI to 5000g of water. (MW: Na 23 g/mol, 0=16 g/mol, Cl=35g/mol) A. 0.000021 m B. 0.02 m C. 0.1081 m D. 0.001081 m 15. 500g of Al:S is dissolved in H20 to make 15L of solution What is its normality? (MW: Al=27g/mol, S=32g/mol, H=lg/mol, O=16g/mol) 0.11 N B. 0.07 N C. 0.22 N D 0.04 N
Chapter4: Calculations Used In Analytical Chemistry
Section: Chapter Questions
Problem 4.31QAP
Related questions
Question
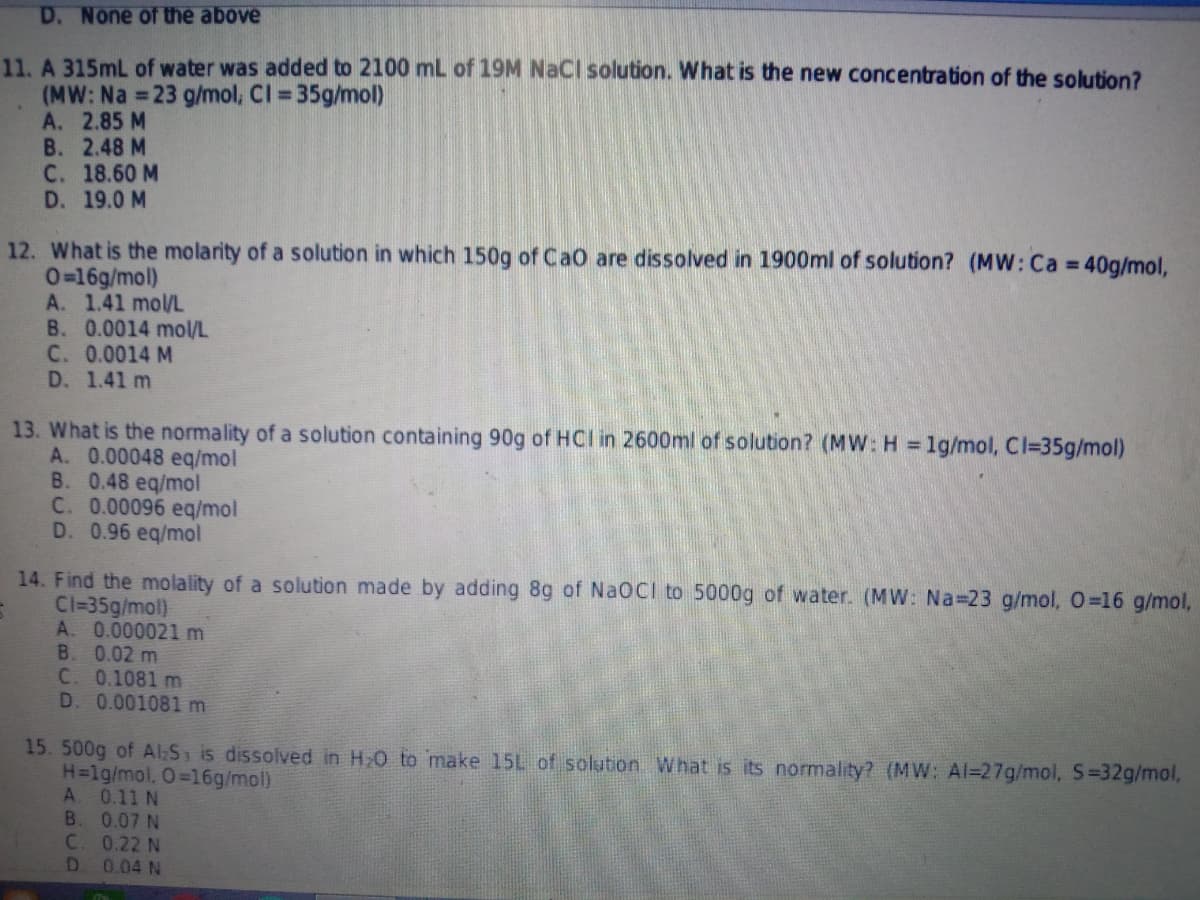
Transcribed Image Text:D. None of the above
11. A 315mL of water was added to 2100 mL of 19M NaCI solution. What is the new concentration of the solution?
(MW: Na 23 g/mol, CI = 35g/mol)
A. 2.85 M
B. 2.48 M
C. 18.60 M
D. 19.0 M
%3D
12. What is the molarity of a solution in which 150g of Ca0 are dissolved in 1900ml of solution? (MW: Ca 40g/mol,
0=16g/mol)
A. 1.41 mol/L
B. 0.0014 mol/L
C. 0.0014 M
D. 1.41 m
13. What is the normality of a solution containing 90g of HCI in 2600ml of solution? (MW: H = 1g/mol, Cl=35g/mol)
A. 0.00048 eq/mol
B. 0.48 eq/mol
C. 0.00096 eq/mol
D. 0.96 eq/mol
14. Find the molality of a solution made by adding 8g of NaOCI to 5000g of water. (MW: Na 23 g/mol, 0=16 g/mol,
Cl=35g/mol)
A. 0.000021 m
B. 0.02 m
C. 0.1081 m
D. 0.001081 m
15. 500g of Al:S is dissolved in H20 to make 15L of solution What is its normality? (MW: Al=27g/mol, S=32g/mol,
H=lg/mol, O=16g/mol)
A
0.11 N
B.
0.07 N
C. 0.22 N
D 0.04 N
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax