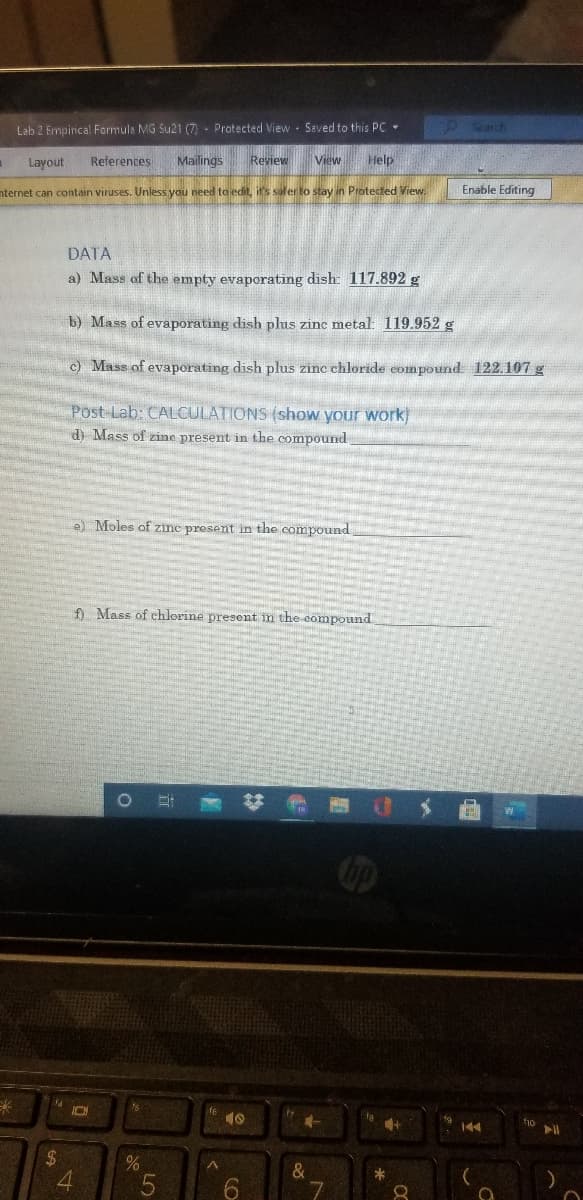
DATA a) Mass of the empty evaporating dish: 117.892 g b) Mass of evaporating dish plus zine metal: 119.952 g c) Mass of evaporating dish plus zinc chloride eompound 122.107 g Post-Lab: CALCULATIONS (show your work) d) Mass of zine present in the compound e) Moles of zine present in the compound f Mass of chlorine present în the compound
DATA a) Mass of the empty evaporating dish: 117.892 g b) Mass of evaporating dish plus zine metal: 119.952 g c) Mass of evaporating dish plus zinc chloride eompound 122.107 g Post-Lab: CALCULATIONS (show your work) d) Mass of zine present in the compound e) Moles of zine present in the compound f Mass of chlorine present în the compound
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 35E: Early tables of atomic weights (masses) were generated by measuring the mass of a substance that...
Related questions
Question
100%
Transcribed Image Text:Lab 2 Empirical Formula MG Su21 (7 - Protected View Saved to this PC
Layout
References
Mailings
Review
View
Help
nternet can contain viruses. Unless you need to edit, it's safer lo stay in Protected View.
Enable Editing
DATA
a) Mass of the empty evaporating dish: 117.892 g
b) Mass of evaporating dish phus zinc metal: 119.952 g
c) Mass of evaporating dish plus zinc chloride compound 122.107 g
Post-Lab: CALCULATIONS (show your work
d) Mass of zine present in the compound
e) Moles of zine present in the compound
f) Mass of chlorine present m the compound
%23
f10
$4
&
6.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning