MOLE-S.I. unit for amount of substance 1 mole is equal to: a. Mass/Grams (perodic table) b. 6.02 times 10^23 c. Liters/Volume (22.4L) Make the following ConveIsions. (If mole is mentioned it's a 1 step problem. If its not mentioned it's 2 steps) 1. 5.6 moles of NaCl to grams 327g of NaCl
MOLE-S.I. unit for amount of substance 1 mole is equal to: a. Mass/Grams (perodic table) b. 6.02 times 10^23 c. Liters/Volume (22.4L) Make the following ConveIsions. (If mole is mentioned it's a 1 step problem. If its not mentioned it's 2 steps) 1. 5.6 moles of NaCl to grams 327g of NaCl
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter3: Composition Of Substances And Solutions
Section: Chapter Questions
Problem 77E: Copper(I) iodide (CuI) is often added to table salt as a dietary source of iodine. How many moles of...
Related questions
Question
It's the 1 mole is equal to one
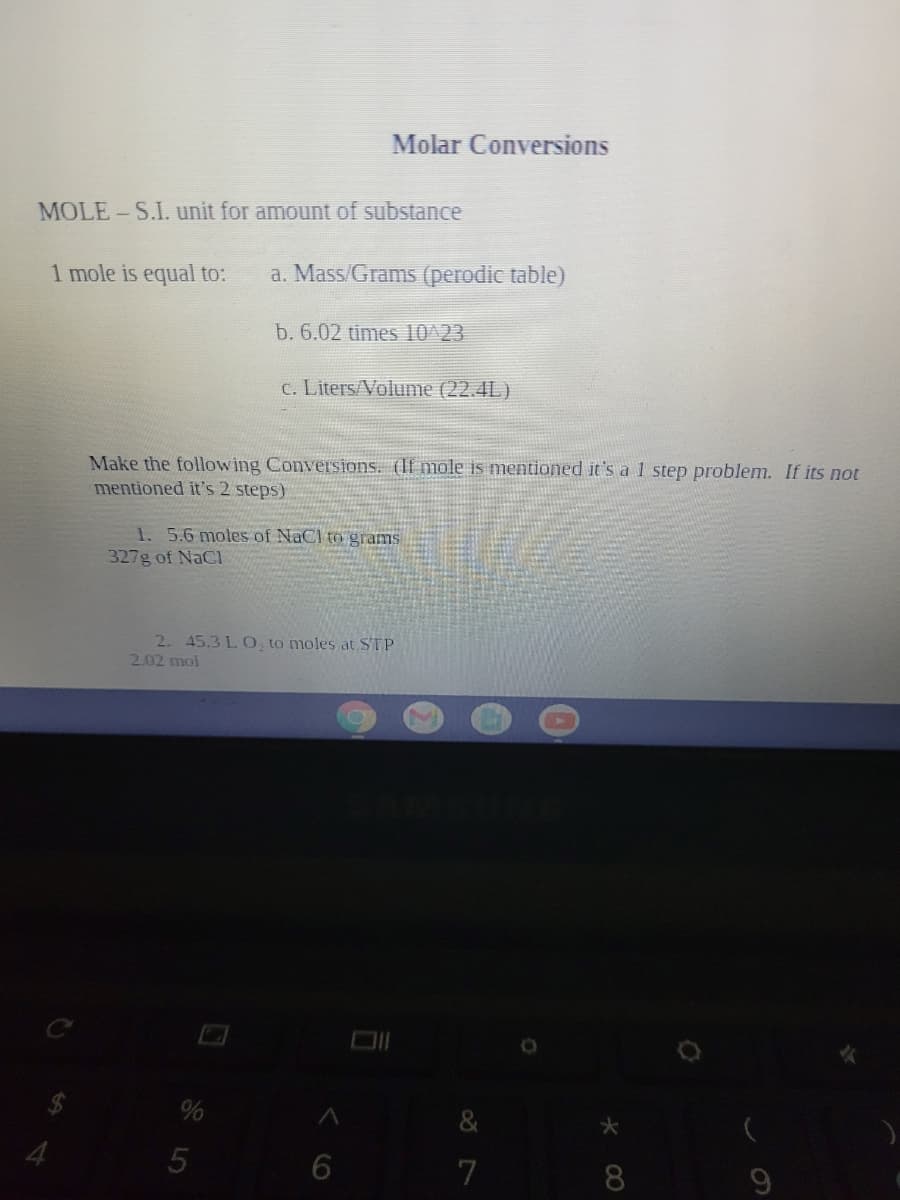
Transcribed Image Text:Molar Conversions
MOLE-S.I. unit for amount of substance
1 mole is equal to:
a. Mass/Grams (perodic table)
b. 6.02 times 10^23
c. LitersVolume (22.4L)
Make the following Conversions. (If mole is mentioned it's a 1 step problem. If its not
mentioned it's 2 steps)
1. 5.6 moles of NaCl to grams
327g of NaCl
2. 45.3 LO, to moles at STP
2.02 mol
4.
7
8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax
